9 Experiment 3: Air Pollution (a day in the life…)
Jennifer Kopanic
Objectives:
1. To bring awareness of the air pollutants that every person contributes to each day.
2. To bring awareness about activities that contribute to air pollution.
3. To determine how to reduce activities that contribute to air pollution.
1. Students learn how daily activities contribute to air pollution.
2. Students use a simple model to investigate air pollution.

Photo from: https://www.britannica.com/explore/savingearth/pollution-overview
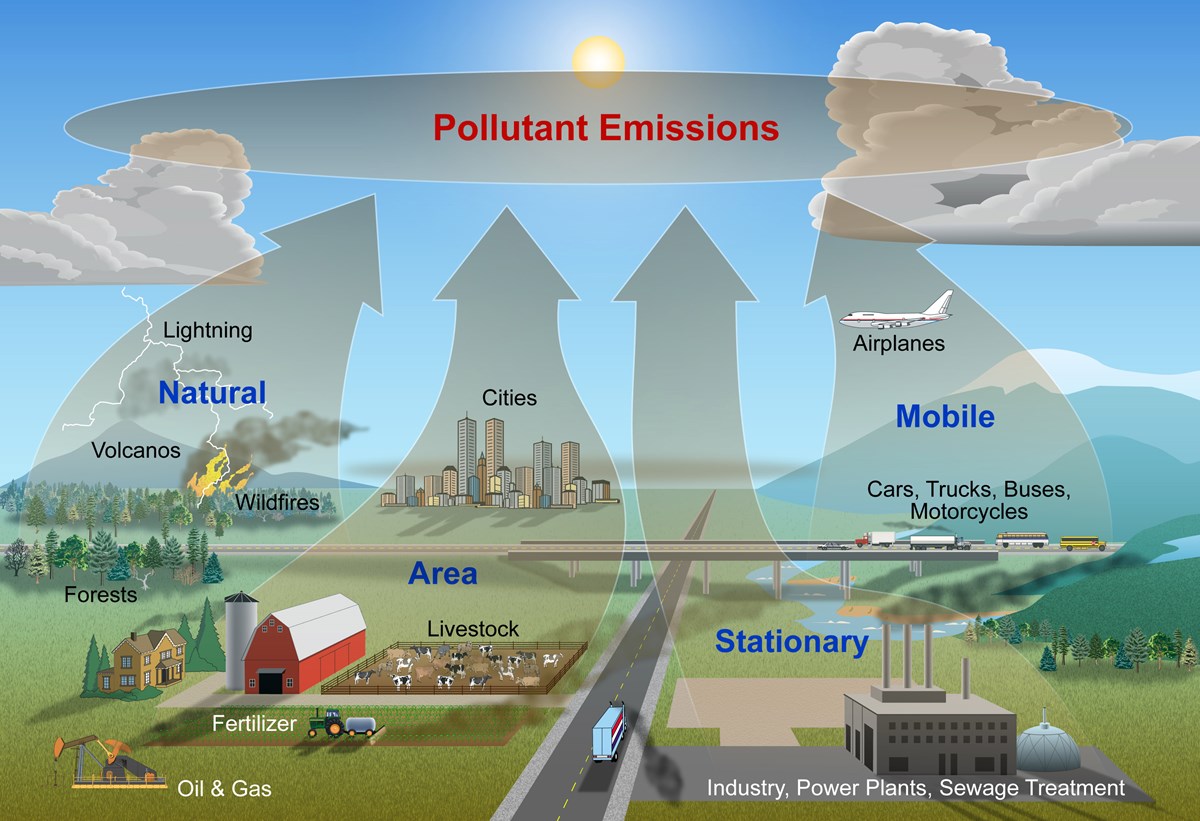
Mobile, stationary, area, and natural sources all emit pollution into the air. Source: https://www.nps.gov/subjects/air/sources.htm
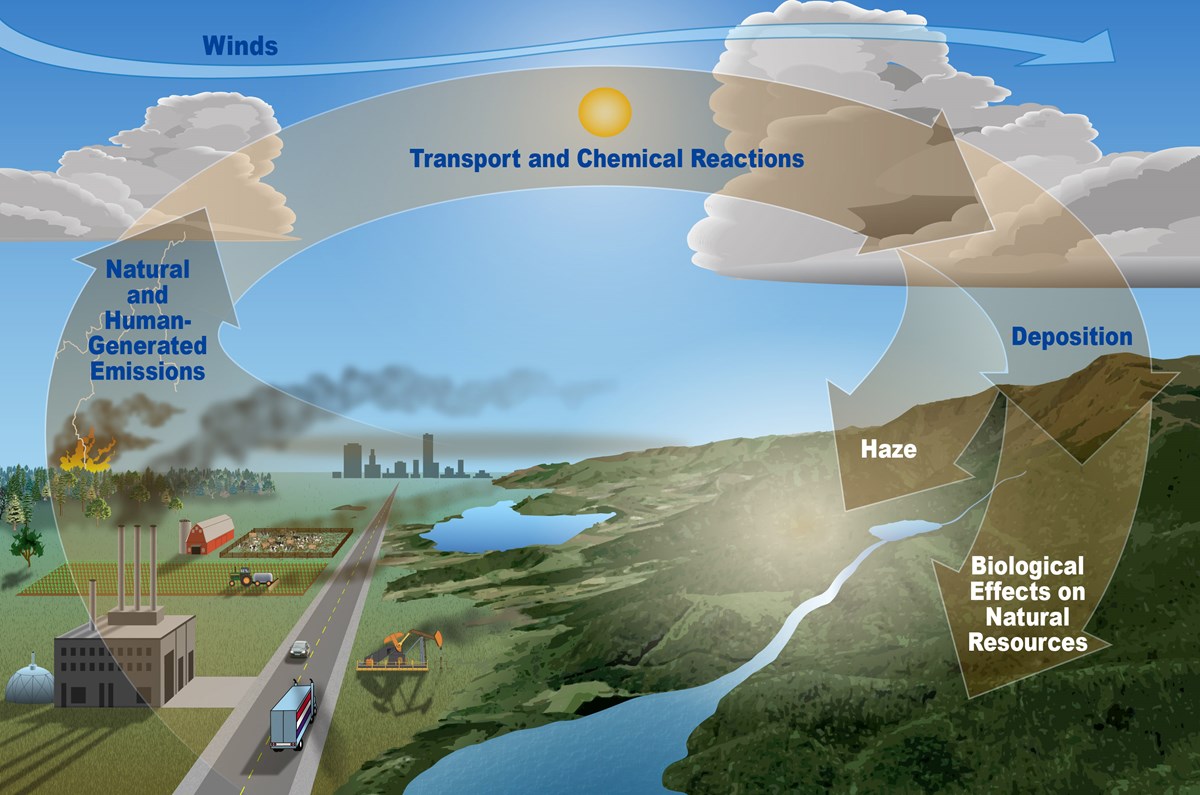
Wind can move air pollutants short or very long distances before they cause harmful impacts. Source: https://www.nps.gov/subjects/air/sources.htm
Materials needed for this experiment:
Water
Beakers
Food coloring (red, yellow, green, and blue)
Sheets of paper to cover the beakers
“Simply stated, an airshed is the volume over an area of land in which airborne chemicals travel to reach a particular river, lake, bay, or other body of water given the area of the land surface.” -From: Ecotoxicology Essentials, 2016
Pre-laboratory questions
- Brainstorm a list of ten sources of air pollution and organize them into four categories:
(A) Gasoline-burning vehicles or engines,
(B) Electricity from fossil fuels, such as coal,
(C) Activities that put particulate matter in the air, such as fires, and
(D) Products that release chemicals into the air. - What is the air feel like if we stand on a beach, what is the air like if we are standing on top of a mountain, or in a forest? What is the air like if we are standing in a big city, near a factory? What is different in the air, where do these things come from? Are they a problem? Why?
There are numerous ways that everyday human activities can contribute to air pollution. These activities may not be immediately apparent as a source of pollution when you consider them from an individual viewpoint. However, the cumulative effect can be profound. This activity attempts to simulate the cumulative effect of various air pollution sources upon the air shed. An air shed is a part of the atmosphere that behaves in a coherent way with respect to the dispersion of emissions. Potential emissions include the following:
• Particulate Matter (PM)
• Volatile Organic Compounds (VOCs)
• Nitrogen Oxides (NOx)
• Sulfur Oxides (SOx)
• Carbon Monoxide (CO)
In this activity, water is used to simulate mixing, which occurs in the air.
• RED food coloring represents vehicle pollution.
• GREEN food coloring represents lawn and garden, motor boat, and construction engines.
• BLUE food coloring represents consumer products and paints.
• YELLOW food coloring represents industry and commercial activities.
• Beakers half full of water represents the air shed.
• Glass stirring rods to stir the water (air shed / atmosphere).
• Students work in pairs.
We’ll be following the day in the life of Scarlett Johansson (playing the role of Natasha Romanoff / Black Widow) in one of the Marvel movies.
24 Hours of Scarlett’s Air Pollution
How this story works: You’ll learn what Scarlett does during her day and how her actions contribute to air pollution. For each hour of the day, there is a part of story and the time, and you’ll decide the source of air pollutants for each activity
While this is a humorous and exaggerated depiction of how someone might excessively contribute air pollutants during their daily activities, every single person does contribute to air pollution on a daily basis.
The water represents their air shed or atmosphere. During the narration, you’ll add a drop of food coloring when you read about something that contributes to air pollution. Have fun with this!
6:00 am – Alarm goes off! Start the day off right with exercising! Spend the first 30 minutes exercising by jumping on the treadmill at home. After a quick job, Scarlett jumps into the shower and get ready for the day.
The water is hot thanks to a water heater that burns natural gas releasing Nitrogen oxides and carbon dioxide into the air. Then, it doesn’t completely burn, natural gas also releases methane. The soap, shampoo, and shaving cream Scarlett uses release Volatile Organic Compounds (VOCs)
into the air. Scarlett can make a lot of air pollution as she showers.
• VOC sources: perfumed soap, shampoo and shaving supplies in the shower; use excessive amount of hot water.
• Towel dry and use deodorant, electric hair dryer, hair spray, perfume, fingernail polish remover and nail polish to fix chipped nail polish on one of her nails.
• CO, NOX, PM2.5, SOX sources: Combustion to heat water.
Add a drop of the appropriate food coloring(s) to your air shed / atmosphere.
7:00 am – Scarlett enjoys eating eggs, oats, and fresh fruit for breakfast. To make the eggs, she uses a stove that uses electricity. Her coffeemaker and toaster use electricity too. The electricity comes from a power plant where coal is burned releasing air pollution, such as carbon dioxide, sulfur dioxide, nitrogen oxides, particulate matter, and mercury. Some coal-fired power plants release lead, carbon monoxide and VOCs too.
The technology that scrubs air pollution from a power plant’s smokestacks is much better than it used to be, but pollution is still released into the air.
Add a drop of the appropriate food coloring(s) to your air shed / atmosphere.
8:00 am – Scarlett gets in her Maserati Quattroporte car and goes to work, speeding all the way (practicing for her role as Black Widow, clearly), except when she is slamming on the breaks. Scarlett doesn’t know that speeding and breaking release more pollution into the air than driving at a steady speed. The burning gasoline releases carbon monoxide, carbon dioxide, nitrogen oxides, particulate matter, and VOCs. On the way, she stops by Starbucks to pick up another coffee drink to prepare for the day. She drives herself to work (does not carpool with co-workers). The the way, she forgets her dry cleaning, goes back home to grab the garments that she needs to take to the dry cleaner, and stops by the dry cleaner before arriving at work.
• VOCs, CO, NOx, SOx, PM sources.
• Cold start in your automobile: High CO emissions.
Add one drop of RED food coloring to your air shed.
9:00 am – Because she was speeding all the way, even with the extra stops, Scarlett arrives at the movie studio early! As she’s waiting for her colleagues to arrive, she lets her car idle as she checks her email and posts a TikTok video about her day so far. Idling the car burns gas, releasing carbon monoxide, carbon dioxide, nitrogen oxides, and VOCs into the air.
• Industrial sources: PM, CO, VOCs, NOx, SOx; manufacturing, mills, construction, space heating; choose any job that might contribute air pollutants.
• Commercial sources: PM, CO, VOCs, NOx, SOx printing, painting, delivery, small manufacturing, dry cleaning.
Add one drop of YELLOW food coloring to your air shed.
9:30 am – Scarlett gets ready with her makeup and wardrobe crew. She gets new make-up done by her staff in her trailer on set. It is a hot day so the AC is cranked up in her trailer. Meanwhile, the crew had used the cheapest paint they can find for painting one of the houses on set. This helps with the overall movie costs. While this might keep costs down, but do they like to know that the cheep paints release VOCs into the air?
10:00 am – Time to start shooting the first scene. In this scene, Scarlett jumps out of a window in the house and rides off on a motorcycle. They need to shoot this scene five times!
12:00 am – It’s lunchtime. Scarlett is excited to take a break. She drives to get nearby fast food (having forgot to pack her lunch) and eats it in her car with the engine running so that the air conditioning is on. Burning gas on the drive and idling in the parking lot releases carbon monoxide, carbon dioxide, nitrogen oxides, particulate matter, and VOCs into the air. The container that her veggie burger came in is made of Styrofoam, also known as polystyrene foam, which releases VOCs into the air. Scarlett races back to the set, speeding the whole way.
• PM, CO, VOCs, NOx, SOx
Add one drop of RED food coloring to your air shed.
1:00 pm – Scarlett is back on set, but this time they are filming a chase scene. Several motorcycles and other vehicles are involved. It takes several hours to film this portion of the movie they are working on.
5:00 pm – Scarlett gets ready to head home. She takes a second showering to get all of the grime, soot, and fake blood off of her from the past few hours of filming. On the way home, Scarlett notices that her car is low on gas. She fills up her gas tank at the hottest point in the day, and notices that her tires look a little low. She decides to fill them later, not realizing that she will use less gasoline if they are inflated properly. Burning more gas releases more air pollution into the air. At the gas station, gasoline vapors are released into the air (which is why gas stations smell like gasoline). At the hottest point in the day, those gasoline vapors are very likely to become the building blocks of ozone, part of smog.
She also remembers that she has to pick up her dress from the dry cleaner. When she went to a fancy party last weekend, she spilled
fancy mustard on her dress. She hopes that the dry cleaner got the stain out. Dry cleaners use products to clean stains that release VOCs and other contaminants into the air.
6:00 pm – As she pulls into her driveway, Scarlett notices that her lawn has been freshly cut. When she had left in the morning, the grass and dandelions were knee high. Scarlett’s landscaper is old school and uses an old but trusty lawnmower that uses gasoline. When mowing Scarlett’s lawn, the lawn mower needed more gasoline. When filling up the lawnmower, the gasoline container spills a little as they pours gasoline into the mower. Vapors get into the air each time they spill gasoline. Scarlett relaxes near her pool on towel that rests the freshly mowed lawn. She puts on aerosol sunscreen to protect her skin – she knows the dangers of the sun and that she needs to protect her skin from UV rays!
7:00 pm – Scarlett lost track of time swimming and sunbathing and hears her stomach rumbling. It is time to make dinner. She pulls a lasagna from her freezer. It was made at a factory where materials (like tomato paste and noodles) are brought in and then energy is used to put them together and package them. The energy used at the lasagna factory came from coal. The ingredients came from all over the world and had to be transported to the factory by trucks and ships burning fossil fuels. Then a frozen foods truck transported the lasagna to a store burning more
gasoline. Scarlett heats up the lasagna in her electric oven, which gets its power from the coal-fired power plant.
8:00 pm – Scarlett binge watches a TV while shopping online for a new pair of shoes. She absolutely loves a pair of shoes, but they are made in ship from India tomorrow. (During the party last weekend, the heel on one of her shoes broke, so she threw them away after finding and ordering a similar pair of shoes.) Running the television and the computer requires power from the coal-fired power plant. Shipping a package from India will burn fossil fuel and release air pollution. And in India, the factory making her shoes will be making air pollution too.
9:00 pm – While it was hot outside today, it’s chilly inside her house. Scarlett starts a fire in the wood-burning fireplace. Burning wood releases particulate matter into the air.
10:00 pm – After cleaning the kitchen with products that release chemicals into the air, Scarlett reads in bed but falls asleep with the light on, using electricity that comes from coal. Scarlett is even making air pollution while she sleeps.
11:00 pm – She is still asleep but she continues to make air pollution because his lights are still on.
12:00 am – She is still asleep but she continues to make air pollution because his lights are still on.
1:00 am – She is still asleep but she continues to make air pollution because his lights are still on.
2:00 am – She is still asleep but she continues to make air pollution because his lights are still on.
3:00 am – Scarlett wakes up and heads to the kitchen for a snack. She is hungry but is also sleepy. While searching her refrigerator for a snack, she dozes off while standing at the door of the refrigerator considering what to eat.
3:30 am – Scarlett wakes up confused. Why am I so cold? she wonders. She realizes that the cold is coming from the refrigerator, which has been open as she dozed off. She no longer wants a snack. She just wants to get back into her warm bed. Edgar turns off the lights, turns on his electric blanket, and drifts off the sleep.
4:00 am – Scarlett is asleep but continues to make air pollution because her electric blanket is on.
5:00 am – Scarlett is asleep but continues to make air pollution because her electric blanket is on.
Time to go home!
Drive home in your car: Almost home and had to turn around to go back to the grocery store.
• PM, CO, VOCs, NOx, SOx
Add one drop of RED food coloring to your air shed.
Stop off to pick up the dry cleaning!
• VOC s
Add one drop of BLUE food coloring to your air shed.
Get ready for the Barbeque!
It’s 4:30 just enough time to mow the yard before sundown.
• PM, CO, VOCs, NOx, SOx
Add one drop of GREEN food coloring to your air shed.
Cookout! The mosquitos are out – spray everyone with the repellant. Get charcoal hot – use lots of igniter fluid. Grill hamburgers.
• PM, CO, VOCs, NOx, SOx
Add one drop of BLUE food coloring to your air shed.
Now look at the air shed in the glass. The original water was clear and pristine.
• What happened to the air shed?
• What contributed to the pollution?
• What actions were unnecessary, careless?
• What would you do differently?
Individuals should evaluate the environmental impacts which result from the choices we make in our everyday activities. When you make a choice
that reduces or eliminates the amount of pollution you contribute to the air you also reduce the need for technologies to remove or recycle
the pollution. Have the students design a sequence which describes their daily activities. Ask the students if they are willing to make one or two lifestyle changes for a semester.
- Pass out the story 24 Hours of Edgar’s Air Pollution and tell students that they are going to walk through a typical day in the life of a person to see the cumulative impact of all air pollution he adds to the atmosphere.
- Have each student read a part of the story (from one hour of Edgar’s day and indicate what type of pollution should be added to the model. Add one drop of the appropriate colors of pollution for each hour. Have students take a closer look at the jar after 24 hours of Edgar’s day. Ask students what colors they see in the jar. Consider what would happen to this air pollution if the air had been moving. Use a spoon to mix the air pollutants. Then ask students what colors they see in the jar.
- Lead a student discussion about how this model atmosphere is like, and unlike the real atmosphere. Ask students to consider how the food coloring is like and unlike air pollution. Have students consider how the model is useful. What does it show well? What does it not show? (Remind students that no model is entirely accurate. All models are representations of something else. This model represents the cumulative impact of air pollutants, but does not reflect how air moves in the atmosphere overall, the chemical changes that happen to air pollution in the air, or the relative amounts of air pollution from different hours of Edgar’s day.)
- Assessment: Have students write their own versions of Edgar’s day that would release less air pollution. Remind students that Edgar needs to do the same activities that he does in the story. Challenge students to write the story so that Edgar does activities in ways that release less air pollution. If time permits, have students research online whether products exist that do not release air pollutants (such as soap, shampoo, paint) and what types of technologies release less air pollution (like renewable energy sources, hybrid or natural gas vehicles).
Here
Have each student read a part of the story (from one hour of Edgar‘s day and indicate what type of pollution should be added to the model.
Add one drop of the appropriate colors of pollution for each hour. Have students take a closer look at the jar after 24 hours of Edgar‘s day.
Ask students what colors they see in the jar. Consider what would happen to this air pollution if the air had been moving. Use a spoon to
mix the air pollutants. Then ask students what colors they see in the jar.
6. Lead a student discussion about how this model atmosphere is like, and unlike the real atmosphere. Ask students to consider how the food
coloring is like and unlike air pollution. Have students consider how the model is useful. What does it show well? What does it not show?
(Remind students that no model is entirely accurate. All models are representations of something else. This model represents the cumulative impact of air pollutants, but does not reflect how air moves in the atmosphere overall, the chemical changes that happen to air pollution
in the air, or the relative amounts of air pollution from different hours of Edgar‘s day.)
7. Assessment: Have students write their own versions of Edgar‘s day that would release less air pollution. Remind students that Edgar needs
to do the same activities that he does in the story. Challenge students to write the story so that Edgar does activities in ways that release
less air pollution. If time permits, have students research online whether products exist that do not release air pollutants (such as soap,
shampoo, paint) and what types of technologies release less air pollution (like renewable energy sources, hybrid or natural gas vehicles)
Air pollution is a broad term that is applied to particulate matter and chemical compounds that are released by humans into the atmosphere and
modify its composition. It was first perceived as a local problem in urban industrialized areas, so factories and power plants started building taller
smoke-stacks. However, taller stacks merely transported the problem elsewhere and soon regional problems such as acid rain were recognized.
In Scandinavia, for example, the acidification of lakes was found to be the result of sulfur dioxide emissions from tall stacks located in central
European countries such as Germany and even in places as far off as Great Britain. More recently, global problems such as climate change and
stratospheric ozone depletion have been widely publicized.
Natural sources that affect atmospheric chemistry include sulfur and nitrogen compounds from volcanoes and biological decay and particulate
matter from dust storms and volcanoes. Plants, trees, and even grasses release volatile organic compounds (VOCs), such as methane, into the air.
Of more concern, since we have the ability to control them, are anthropogenic (human-made) air pollutants, such as carbon monoxide, sulfur dioxide, some fraction of VOCs and nitrogen oxides. The largest source of anthropogenic pollution is the burning of fossil fuels, including coal, oil,
and gas, in our homes, factories, and vehicles.
There are many forms of air pollution that are human-made. Industrial plants, combustion-fired power plants and vehicles with internal combustion
engines generate nitrogen oxides, VOCs, carbon monoxide, carbon dioxide, sulfur dioxide and particulates. In many cities cars are the primary
source of air pollutants. Stoves and incinerators, especially ones that are coal or wood-fired, and farmers burning their crop waste produce
carbon monoxide, carbon dioxide, as well as particulates. Other human-made sources include aerosol sprays and gases leaking from refrigeration
systems, as well as fumes from paint, varnish, and other solvents. Additional pollutants, like ozone and acids, are made in the atmosphere when
human-made gases combine chemically.
Air pollution doesn’t stay in one place. Winds and weather play an important part in transport of pollution locally, regionally, and even around the
world.
FOSTERING DISCUSSION
Consider following this activity with a project in which students record their daily activities that cause air pollution and having students make one
or two lifestyle changes that would decrease air pollution.
Some variations to consider – might the jar be hidden behind a piece of paper ( or wrapped) while they add the drops as they go through their
“audit”.. and then reflect on what the result might look like BEFORE unveiling the jar.
it is even possible that the students could add – choose to add beneficial actions – things that would counter the effects — plant trees, fit better
technology to cars, factories etc — This might be done by adding a little BLEACH to the jars – CARE NEEDS TO BE TAKEN as bleach should not be
handled by young students, but might be added by a supervisor/facilitator during a discussion about WHAT WE CAN DO to HELP.. The bleach will
( over a few minutes) bleach the food dyes, making the colour lighter.. different food colours will bleach differently ( you must check before hand)
red and blue and green will often become colourless, or light yellow).
POSSIBLE EXTENSIONS
Bibliotheca Alexandrina Planetarium Science Center
—–
Have each student read a part of the story (from
one hour of Edgar’s day and indicate what type
of pollution should be added to the model. Add
one drop of the appropriate colors of pollution
for each hour. Have students take a closer look at
the jar after 24 hours of Edgar’s day. Ask studentswhat colors they see in the jar. Consider what
would happen to this air pollution if the air had
been moving. Use a spoon to mix the air pollutants. Then ask students what colors they see in
the jar.
Lead a student discussion about how this model
atmosphere is like, and unlike the real atmosphere. Ask students to consider how the food
coloring is like and unlike air pollution. Have
students consider how the model is useful. What
does it show well? What does it not show? (Remind students that no model is entirely accurate.
All models are representations of something
else. This model represents the cumulative impact of air pollutants, but does not reflect how
air moves in the atmosphere overall, the chemical changes that happen to air pollution in the
air, or the relative amounts of air pollution from
different hours of Edgar’s day.)
Assessment: Have students write their own versions of Edgar’s day that would release less air
pollution. Remind students that Edgar needs to
do the same activities that he does in the story.
Challenge students to write the story so that
Edgar does activities in ways that release less air
pollution. If time permits, have students research
online whether products exist that do not release
air pollutants (such as soap, shampoo, paint) and
what types of technologies release less air pollution (like renewable energy sources, hybrid or
natural gas vehicles).
Air pollution is a broad term that is applied to particulate
matter and chemical compounds that are released by humans into the atmosphere and modify its composition. It
was first perceived as a local problem in urban industrialized areas, so factories and power plants started building
taller smoke-stacks. However, taller stacks merely transported the problem elsewhere and soon regional problems such as acid rain were recognized. In Scandinavia,
for example, the acidification of lakes was found to be the
result of sulfur dioxide emissions from tall stacks located
in central European countries such as Germany and
even in places as far off as Great Britain. More recently,
global problems such as climate change and stratospheric ozone depletion have been widely publicized.
Natural sources that affect atmospheric chemistry include
sulfur and nitrogen compounds from volcanoes and biological decay and particulate matter from dust storms and
volcanoes. Plants, trees, and even grasses release volatile
organic compounds (VOCs), such as methane, into the air.
Of more concern, since we have the ability to control them,
are anthropogenic (human-made) air pollutants, such as
carbon monoxide, sulfur dioxide, some fraction of VOCs
and nitrogen oxides. The largest source of anthropogenic
pollution is the burning of fossil fuels, including coal, oil,
and gas, in our homes, factories, and vehicles.
There are many forms of air pollution that are humanmade. Industrial plants, combustion-fired power plants
and vehicles with internal combustion engines generate nitrogen oxides, VOCs, carbon monoxide, carbon
dioxide, sulfur dioxide and particulates. In many cities
cars are the primary source of air pollutants. Stoves and
incinerators, especially ones that are coal or wood-fired,
and farmers burning their crop waste produce carbon
monoxide, carbon dioxide, as well as particulates. Other
human-made sources include aerosol sprays and gases
leaking from refrigeration systems, as well as fumes from
paint, varnish, and other solvents. Additional pollutants,
like ozone and acids, are made in the atmosphere when
human-made gases combine chemically.
Fostering Discussions Air pollution doesn’t stay in one place. Winds and weather play an important part in transport of pollution locally,
regionally, and even around the world.
Consider following this activity with a project in which students record their daily activities that cause air pollution
and having students make one or two lifestyle changes
that would decrease air pollution.
Some variations to consider – might the jar be hidden
behind a piece of paper ( or wrapped) while they add the
drops as they go through their “audit”.. and then reflect on
what the result might look like BEFORE unveiling the jar.
it is even possible that the students could add – choose to
add beneficial actions – things that would counter the effects — plant trees, fit better technology to cars, factories
etc — This might be done by adding a little BLEACH to
the jars – CARE NEEDS TO BE TAKEN as bleach should
not be handled by young students, but might be added by
a supervisor/facilitator during a discussion about WHAT
WE CAN DO to HELP.. The bleach will ( over a few
minutes) bleach the food dyes, making the colour lighter..
different food colours will bleach differently ( you must
check before hand) red and blue and green will often
become colourless, or light yellow).
Possible Extensions
Authors/Source
Bibliotheca Alexandrina Planetarium Science Center
Background
Air pollution is a broad term that is applied to particulate matter and chemical compounds that are released by humans into the atmosphere and modify its composition. It was first perceived as a local problem in urban industrialized areas, so factories and power plants started building taller smokestacks. However, taller stacks merely transported the problem elsewhere and soon regional problems such as acid rain were recognized. In Scandinavia, for example, the acidification of lakes was found to be the result of sulfur dioxide emissions from tall stacks located in central European countries such as Germany and even in places as far off as Great Britain. More recently, global problems such as climate change and stratospheric ozone depletion have been widely publicized.
Natural sources that affect atmospheric chemistry include sulfur and nitrogen compounds from volcanoes and biological decay and particulate matter from dust storms and volcanoes. Plants, trees, and even grasses release volatile organic compounds (VOCs), such as methane, into the air. Of more concern, since we have the ability to control them, are anthropogenic (human-made) air pollutants, such as carbon monoxide, sulfur dioxide, some fraction of VOCs, and nitrogen oxides. The largest source of anthropogenic pollution is the burning of fossil fuels, including coal, oil, and gas, in our homes, factories, and vehicles.
There are many forms of air pollution that are human-made. Industrial plants, combustion-fired power plants, and vehicles with internal combustion engines generate nitrogen oxides, VOCs, carbon monoxide, carbon dioxide, sulfur dioxide, and particulates. In many cities, cars are the primary source of air pollutants. Stoves and incinerators, especially ones that are coal or wood-fired, and farmers burning their crop waste produce carbon monoxide, carbon dioxide, as well as particulates. Other human-made sources include aerosol sprays and gases leaking from refrigeration systems, as well as fumes from paint, varnish, and other solvents. Additional pollutants, like ozone and acids, are made in the atmosphere when human-made gases combine chemically.
Air pollution doesn’t stay in one place. Winds and weather play an important part in transport of pollution locally, regionally, and even around the world.
Extension
- Consider following this activity with a project in which students record their daily activities that cause air pollution and having students make one or two lifestyle changes that would decrease air pollution.
Credits
This version of Whirling, Swirling Air Pollution by Lisa Gardiner is based on an activity with the same name by Donna Rogers at the US EPA.
Experiment is adapted from the Air and Waste Management Association Environmental Resource Guide for Air Quality.
Adapted from © 2021 UCAR Center for Science Education. • scied.ucar.edu


Feedback/Errata