Objective 5c: Search ClinicalTrials.gov (optional, search 6)
Your group may choose to search ClinicalTrials.gov. A ClinicalTrials.gov search is not a required part of the group assignment.
_______________________________________________________________________
Why Would you Choose to run A Clinical.Trials.gov search?
- Almost all trials conducted in the United States during recent years (and many conducted around the world) will be registered in ClinicalTrials.gov. So a search of this database can give you an idea of the research that has been conducted.
- You could use the ClinicalTrials.gov list as the starting place for organizing your PubMed search results. Several journal articles may be produced from the analyses conducted using a single trial's data.
- If you were a practicing pharmacist, you might want to determine whether all completed trials had reported their results. A completed trial that hasn't reported results should cause some concern, especially when the trial without reported results was sponsored by the drug manufacturer.
_______________________________________________________________________
Running a ClinicalTrials.gov Search
- Go to clinicaltrials.gov (Opens in a new window). Drag the new tab/window onto your working window.
- Enter the name of disease of interest (paroxysmal nocturnal hemoglobinuria) in the "Condition/disease" box. Make an appropriate choice from the list of conditions that appears.
- Repeat this process for the "Other terms" box (enter "thrombosis") and the "Intervention/treatment" box (enter "eculizumab") .
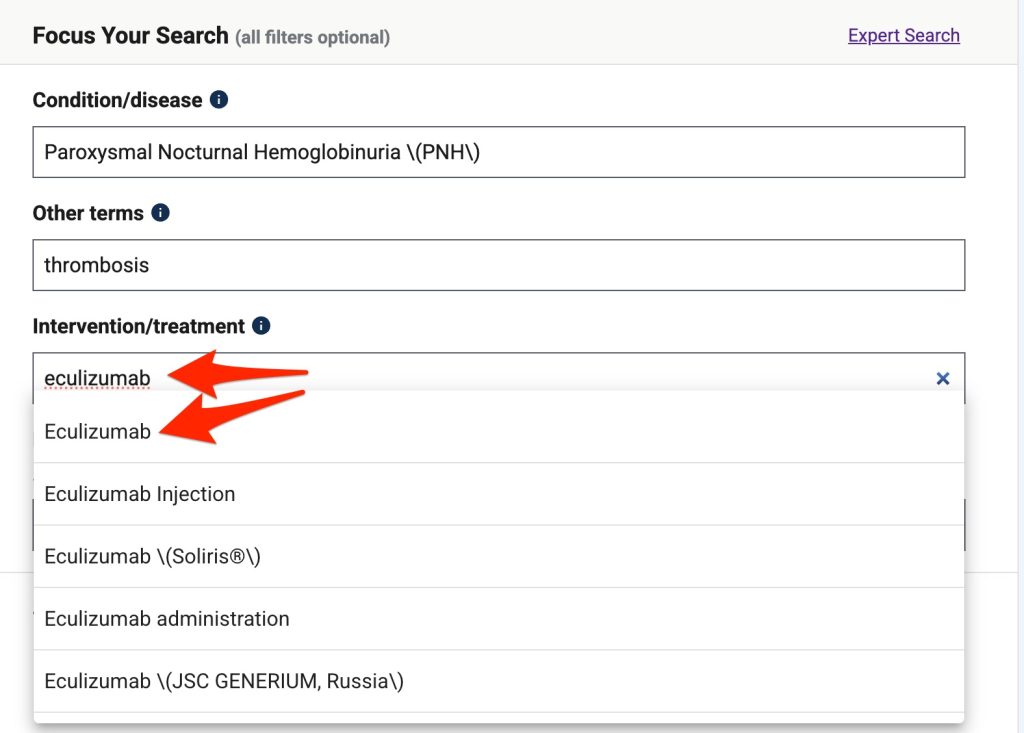
- Click the "Search" button. Results will appear.
- A variety of options are available for downloading either all or selected results.
_______________________________________________________________________
Documenting a ClinicalTrials.gov Search
- Simply type the name of each box and the option chosen for each box.
- Include the number of results retrieved on a separate line.
Search Strategies:
1 MeSH search for indexed PubMed (MEDLINE) records for Phase II-IV and randomized controlled trials
((("eculizumab" [Supplementary Concept]) AND "Hemoglobinuria, Paroxysmal"[Mesh]) AND "Embolism and Thrombosis"[Mesh]) AND "therapy" [Subheading] AND (clinicaltrialphaseii[Filter] OR clinicaltrialphaseiii[Filter] OR clinicaltrialphaseiv[Filter] OR randomizedcontrolledtrial[Filter]) AND (humans[Filter]) AND (english[Filter])
3 results
2 (optional). Keyword search for indexed PubMed (MEDLINE) records for Phase II-IV and randomized controlled trials** that cannot be identified by the MeSH search.
(Eculizumab* OR 219685-50-4 OR h5G1.1* OR h5g11 OR h5G1-1 OR 5G1.1 OR Soliris OR Elizaria OR Alexion OR Acveris OR Bekemv OR Epysqli) AND (((intermittent OR paroxysmal ) AND ( haemoglobinur* OR hemoglobinur* OR haematur* OR hematur* OR haematinur* OR hematinur*)) OR PNH OR Marchiafava-Micheli [tiab]) AND (Clot OR Clots OR budd-chiari[tiab] OR embol* OR postthromb* OR thromb*) AND ((clinicaltrialphaseii[Filter] OR clinicaltrialphaseiii[Filter] OR clinicaltrialphaseiv[Filter] OR randomizedcontrolledtrial[Filter]) AND (humans[Filter]) AND (1800/1/1:2005/1/1[pdat]) AND (english[Filter]))
0 results
3 Keyword search for unindexed PubMed records for randomized controlled trials**.
(Eculizumab* OR 219685-50-4 OR h5G1.1* OR h5g11 OR h5G1-1 OR 5G1.1 OR Soliris OR Elizaria OR Alexion OR Acveris OR Bekemv OR Epysqli) AND (((intermittent OR paroxysmal ) AND ( haemoglobinur* OR hemoglobinur* OR haematur* OR hematur* OR haematinur* OR hematinur*)) OR PNH OR Marchiafava-Micheli [tiab]) AND (Clot OR Clots OR budd-chiari[tiab] OR embol* OR postthromb* OR thromb*) NOT MEDLINE[sb] AND random* AND (english[Filter])
3 results
4. Keywords search for unindexed and indexed PubMed records for meta-analyses, observational and economic studies, and guidelines:
(Eculizumab* OR 219685-50-4 OR h5G1.1* OR h5g11 OR h5G1-1 OR 5G1.1 OR Soliris OR Elizaria OR Alexion OR Acveris OR Bekemv OR Epysqli) AND (((intermittent OR paroxysmal ) AND ( haemoglobinur* OR hemoglobinur* OR haematur* OR hematur* OR haematinur* OR hematinur*)) OR PNH OR Marchiafava-Micheli [tiab]) AND (Clot OR Clots OR budd-chiari[tiab] OR embol* OR postthromb* OR thromb*) AND (english[Filter]) AND (consensusdevelopmentconference[Filter] OR consensusdevelopmentconferencenih[Filter] OR guideline[Filter] OR meta-analysis[Filter] OR practiceguideline[Filter] OR observationalstudy[Filter] “Cost-Benefit Analysis”[Mesh] OR “Cost-Effectiveness Analysis”[Mesh] OR “Economics, Pharmaceutical”[Mesh] OR guideline*[ti] OR consensus[ti] OR “position statement*”[ti] OR meta-analysis[ti] OR observational[tiab] OR case-control*[tiab] OR cohort*[tiab] OR pharmacoeconomic*[tiab] OR cost-benefit*[tiab] OR cost-utility[tiab] OR cost-effectiveness[tiab] )
8 results
5 (optional) EMBASE search for records for Phase II-IV and randomized controlled trials:
#6
#5 AND ('clinical practice guideline'/de OR 'meta analysis'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'practice guideline'/de OR 'randomized controlled trial'/de)
25 results
#5
#3 NOT ('chapter'/it OR 'clinical trial'/it OR 'conference abstract'/it)
291
#4
#3 AND ('chapter'/it OR 'clinical trial'/it OR 'conference abstract'/it)
614
#3
(eculizumab*:ab,kw,ti OR '219685 50 4':ab,kw,ti OR h5g1.1*:ab,kw,ti OR h5g11:ab,kw,ti OR 'h5g1 1':ab,kw,ti OR 5g1.1:ab,kw,ti OR soliris:ab,kw,ti OR elizaria:ab,kw,ti OR alexion:ab,kw,ti OR acveris:ab,kw,ti) AND ((intermittent:ab,kw,ti OR paroxysmal:ab,kw,ti) AND (haemoglobinur*:ab,kw,ti OR hemoglobinur*:ab,kw,ti OR haematur*:ab,kw,ti OR hematur*:ab,kw,ti OR haematinur*:ab,kw,ti OR hematinur*:ab,kw,ti) OR pnh:ab,kw,ti OR 'marchiafava micheli':ab,kw,ti) AND (clot:ab,kw,ti OR clots:ab,kw,ti OR 'budd chiari':ab,kw,ti OR embol*:ab,kw,ti OR postthromb*:ab,kw,ti OR thromb*:ab,kw,ti) AND [humans]/lim AND [english]/lim
905
#2
(eculizumab* OR '219685 50 4' OR h5g1.1* OR h5g11 OR 'h5g1 1' OR 5g1.1 OR soliris OR elizaria OR alexion OR acveris) AND ((intermittent OR paroxysmal) AND (haemoglobinur* OR hemoglobinur* OR haematur* OR hematur* OR haematinur* OR hematinur*) OR pnh OR 'marchiafava micheli') AND (clot OR clots OR 'budd chiari' OR embol* OR postthromb* OR thromb*) AND [humans]/lim AND [english]/lim
1,346
#1
(eculizumab* OR '219685 50 4' OR h5g1.1* OR h5g11 OR 'h5g1 1' OR 5g1.1 OR soliris OR elizaria OR alexion OR acveris) AND ((intermittent OR paroxysmal) AND (haemoglobinur* OR hemoglobinur* OR haematur* OR hematur* OR haematinur* OR hematinur*) OR pnh OR 'marchiafava micheli') AND (clot OR clots OR 'budd chiari' OR embol* OR postthromb* OR thromb*)
1,461
6 (optional): ClinicalTrials.gov search
Disease/Condition:Paroxysmal Nocturnal Hemoglobinuria \(PNH\)
Other terms: thrombosis
Intervention/treatment: Eculizumab
16 results
The following drug names were omitted from the keyword search strategies 2- 4 because they were used frequently to indicate discussion of irrelevant concepts and were causing retrieval of irrelevant results: not applicable
** If you are studying a rare disease and find few if any randomized controlled trials, search for any clinical trial instead of randomized controlled trials only and change the template headings accordingly.
