Objective 4d: Searching Drugs@FDA (optional, search 6)
Why?
You may wish to see the trial descriptions submitted to the FDA before ‘your’ drug’s approval.
How?
- Go to the Drugs@FDA search site
- Search for your drug’s name (either English-generic name or a US brand name)
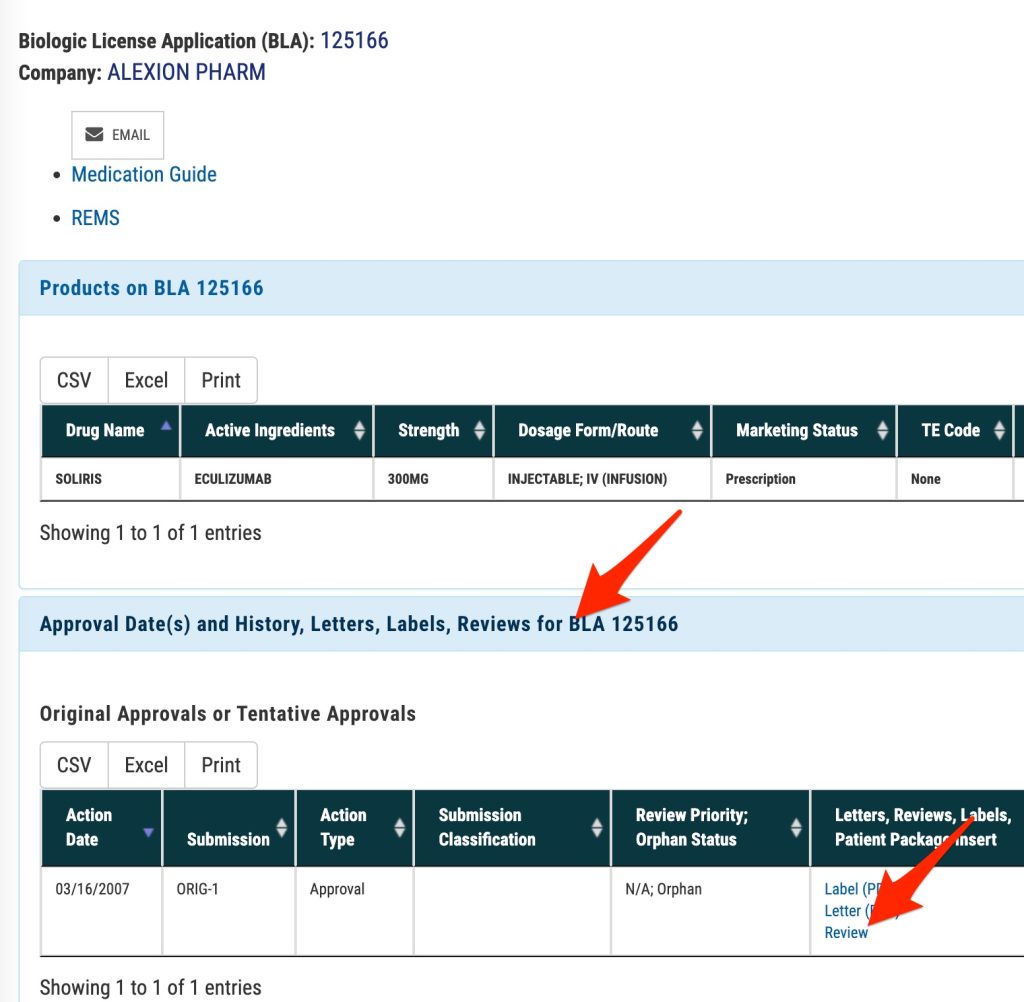
- Click on the “Approval Date(s) and History, Letters, Labels, Reviews for ___” link
- You should then see (and be able to click) the “Review” link. You may also wish to look at the most recent “label” as this may also contain trial descriptions.
Document the search
- If your group performs a Drugs@FDA search, type the drug name used for the search under the “Drugs@FDA” heading.
Search Strategies:
1. MeSH search for indexed PubMed (MEDLINE) records for clinical trials.
(((“eculizumab” [Supplementary Concept]) AND “Hemoglobinuria, Paroxysmal”[Mesh]) AND “Embolism and Thrombosis”[Mesh]) AND “therapy” [Subheading] AND ((clinicaltrial[Filter]) AND (english[Filter]))
3 results
(If you decided to conduct a combined MeSH-keyword search for indexed records, it would replace the search above , and you would indicate the number of results it retrieved.)
2 Keyword search for unindexed PubMed records (PREMEDLINE, OLD MEDLINE, PMC)
(Eculizumab* OR 219685-50-4 OR h5G1.1* OR h5g11 OR h5G1-1 OR 5G1.1 OR Soliris OR Elizaria OR Alexion OR Acveris) AND (((intermittent OR paroxysmal ) AND ( haemoglobinur* OR hemoglobinur* OR haematur* OR hematur* OR haematinur* OR hematinur*)) OR PNH OR Marchiafava-Micheli [tiab]) AND (Clot[tiab] OR Clots[tiab] OR budd-chiari[tiab] OR embol* OR postthromb* OR thromb*) NOT MEDLINE[sb] AND (english[Filter])
71 results
3. Keyword search for unindexed and indexed PubMed records for meta-analyses: (optional)
(Eculizumab* OR 219685-50-4 OR h5G1.1* OR h5g11 OR h5G1-1 OR 5G1.1 OR Soliris OR Elizaria OR Alexion OR Acveris) AND (((intermittent OR paroxysmal ) AND ( haemoglobinur* OR hemoglobinur* OR haematur* OR hematur* OR haematinur* OR hematinur*)) OR PNH OR Marchiafava-Micheli [tiab]) AND (Clot OR Clots[tiab] OR budd-chiari[tiab] OR embol* OR postthromb* OR thromb*) AND (english[Filter]))
Applied: “systematic rev _ Meta-analyses + guidelines” filter
11 results
4. Search of ClinicalTrials.gov
(Eculizumab* OR 219685-50-4 OR h5G1.1* OR h5g11 OR h5G1-1 OR 5G1.1 OR Soliris OR Elizaria OR Alexion OR Acveris) AND (((intermittent OR paroxysmal ) AND ( haemoglobinur* OR hemoglobinur* OR haematur* OR hematur* OR haematinur* OR hematinur*)) OR PNH OR Marchiafava-Micheli) AND (Clot OR Clots OR budd-chiari OR embol* OR postthromb* OR thromb*)
No filters
4 results
5. EMBASE (optional):
| No. | Query | Results | Date |
| #11 | #10 AND (‘clinical trial’/de OR ‘controlled clinical trial’/de OR ‘non inferiority trial’/de OR ‘phase 1 clinical trial’/de OR ‘phase 2 clinical trial’/de OR ‘phase 3 clinical trial’/de OR ‘randomized controlled trial’/de) | 16 | 13-Aug-24 |
| #10 | #8 NOT (‘conference abstract’/it OR ‘letter’/it OR ‘review’/it OR ‘tombstone’/it) | 76 | 13-Aug-24 |
| #8 | (eculizumab*:ti OR ‘219685 50 4’:ti OR h5g1.1*:ti OR h5g11:ti OR ‘h5g1 1’:ti OR 5g1.1:ti OR soliris:ti OR elizaria:ti OR alexion:ti OR acveris:ti) AND ((((intermittent OR paroxysmal) NEXT/4 (haemoglobinur* OR hemoglobinur* OR haematur* OR hematur* OR haematinur* OR hematinur*)):ab,kw,ti) OR pnh:ab,kw,ti OR ‘marchiafava micheli’:ab,kw,ti) AND (clot:ab,ti,kw OR clots:ab,ti,kw OR ‘budd chiari’:ab,ti,kw OR embol*:ab,ti,kw OR postthromb*:ab,ti,kw OR thromb*:ab,ti,kw) AND [humans]/lim AND [english]/lim | 291 | 13-Aug-24 |
6. Drugs@FDA (optional):
eculizumab
The following drug names were omitted from the keyword search strategies 2- 4 because they were used frequently to indicate discussion of irrelevant concepts and were causing retrieval of irrelevant results: (None)
