6 Measuring sugar concentration using Skittles
The Science of Sugar Concentration and Skittles

Cost: Less than $6.00 Difficulty: Low Time: Less than an hour Continuous: No.
Summary: Teach students about substance concentrations, solvents, solutes, how things dissolve, etc.
ELO’s – Concentrates, Saturation, solutions, dissolving, solute, solvent, chemistry, molecules, observation, osmosis, discussion, building a hypothesis, critical thinking
Supplies needed:
- Foam plates (one per student)
- Water source (with cup)
- A bag of Skittles (3 different colored skittles per plate)
- Sugar cubes
- A mess bucket and maybe cleaning materials for afterwards.
- Scrap newspaper (to cover desks if necessary)
Pro-tip: If your students are prone to eat the Skittles or sugar cubes, make sure you have enough on hand.
Activity
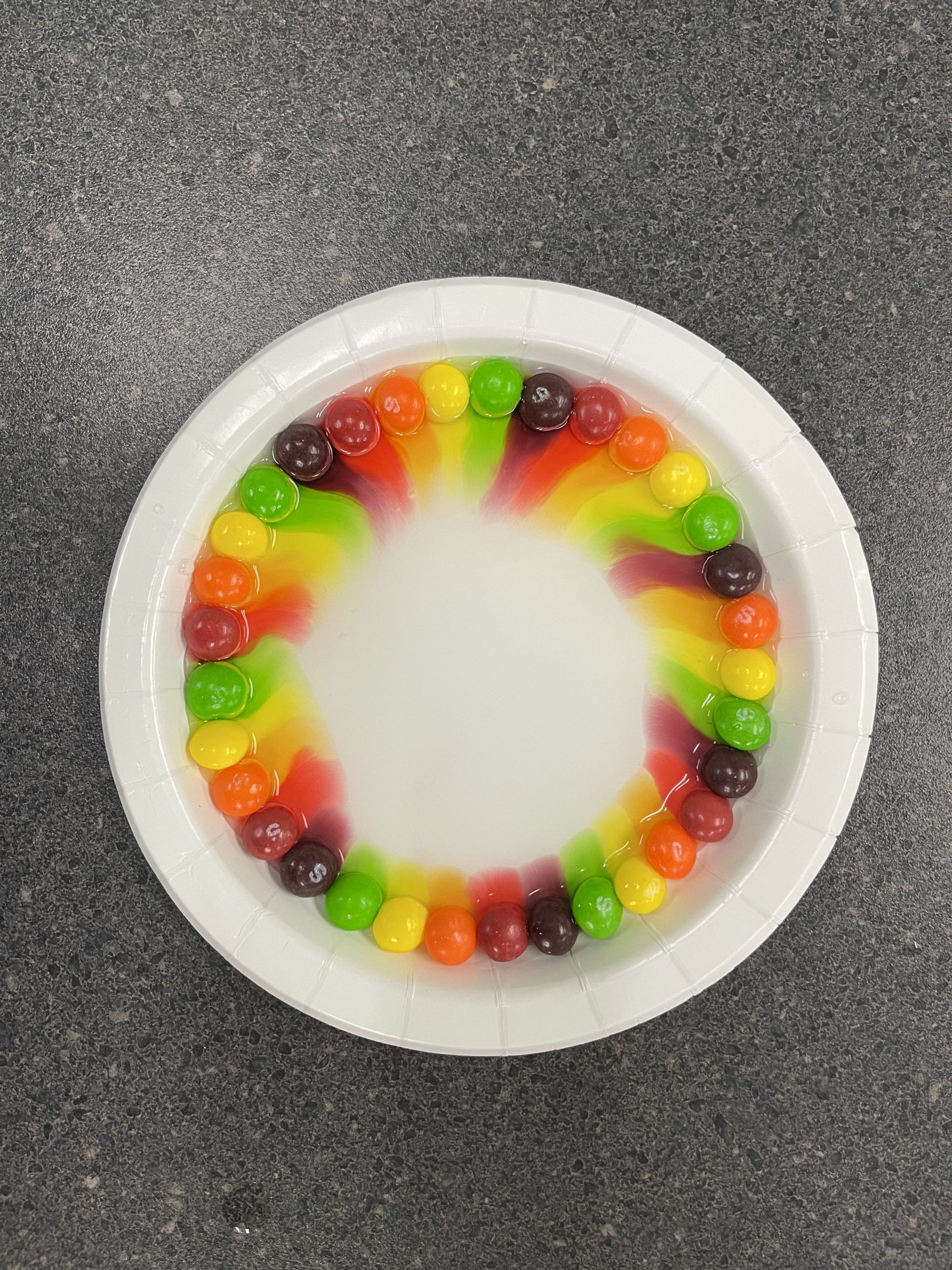
- Fill your shallow foam plate with water (skittles only need to be half covered with water).
- Arrange the skittles in the center forming a triangle with each of them about an inch or two apart.
- Do not disturb the skittles or the water; allowing the colors to begin to spread.
- Have the students observe their plates. Ask the students what happened? [The colors meet equally in the center of the plate.
- When the three colors meet in the middle and begin to work their way outward, have the students make another hypothesis (guess). If you put a sugar cube in the center of the plate, between the skittles, what will happen? Will the color push inward or push outward?
- Place a single sugar cube in the center of the skittles and allow everything to dissolve.
- Have the students observe again.
- Have the students explain what happened? Why did it happen?
Discussion
This activity covers a fundamental principle of chemistry – molecules move from higher concentrations to lower concentrations; meaning that they disperse over time. This is called a concentration gradient. It relates to everything from the smell of a freshly baked apple pie filling the house to concentrated sugar spreading out in water on your plate.
When you first add the skittles they begin dissolving in the water; sending the food coloring outwards as this happens. The reason that the food coloring meets in the middle of the plate and doesn’t mix is because each food color has the same amount of sugar dissolved from each skittle. Once you add the pure sugar to the center of the food coloring intersection, the sugar cube begins to dissolve as well. Now most of sugar is found in the center of the plate and the least amount of sugar is found at the edge of the plate, the area containing mostly plain water. As the sugar cube dissolves, it pushed outwards into the rest of the solution, sending the colored water outwards as well.
As a teacher, there are a number of opportunities during this activity to ask your students for a hypothesis or answers. They could try changing the variables and attempt to predict a different outcome (ex, hot water vs cold water or different colored skittles or different sugar types).
Also, try adding hot water to a ring of skittles around the edge of the plate!
