4 Hosts, Reservoirs, and Vectors
Matthew G. Bolek; Kyle D. Gustafson; and Gabriel J. Langford
Introduction
From the parasite’s perspective, a host represents a resource and a habitat where the parasite can grow and reproduce. Once produced, reproductive stages of the parasite must find their way back to infect another host. Unlike most free-living organisms, one of the major challenges for a parasite is to continuously encounter and colonize suitable hosts for the propagation of the next generation in the life cycle. From a statistical point of view, any individual parasitic organism has an exceedingly low probability of transferring from one host to another. Indeed, the spatial and temporal difficulties parasites face to complete their life cycle must be overcome by enormous reproductive outputs and/or by exploiting complex ecological associations between successive hosts (Tinsley, 1990).
For any parasite transmission event to occur, an infective stage of a parasite has to first encounter a potential host. This challenge can be considered an encounter filter (Euzet and Combes, 1980). For example, ecological conditions will affect the spatial and temporal overlap of host and parasite populations and species-specific behavioral characteristics can bridge or reduce encounters between parasites and their hosts. Adaptations that increase encounter rates with potential hosts will likely lead to higher infection probabilities (Combes, 2005). Following the encounter; however, another hurdle must be cleared which can be thought of as a compatibility filter, and this must be overcome for a parasite infection to become established. In this case, and after encountering a potential host, the compatibility filter determines whether the parasite is able to survive, grow, or reproduce in the host. For example, a parasite might be able to infect a variety of different species of potential hosts, but most of those species would not possess the necessary resources for the parasite to survive. Even when appropriate hosts are encountered, host susceptibility to the parasite is controlled by a variety of host factors such as genetics, immunity, and physiology, among others (Combes, 2005). To overcome these challenges, parasites have evolved various types of life cycles, which include different types and combinations of hosts used for multiplication, growth, reproduction, and/or transmission.
The Role of Hosts in Life Cycles and Transmission of Parasites
Parasitologists differentiate among various types of hosts based on the specific roles those hosts play in the development, reproduction, and transmission of the parasite. In a typical life cycle, a host in which a parasite reaches sexual maturity and reproduces is known as the definitive host. In contrast, an intermediate host is one that is required for parasite development, but one in which the parasite does not reach sexual maturity. In most cases, the parasite goes through morphological and developmental changes in an intermediate host. In some cases, the parasite increases in numbers within an intermediate host. For example, all species of digenetic trematodes and some species of cestodes increase in number in the intermediate host through an asexual process known as polyembryony, the formation of more than one embryo from a single zygote (Craig et al., 1997). As a result of polyembryony, intermediate hosts can play a major role in increasing the probability of parasites encountering the next host in the life cycle.
A paratenic host, or transport host, is one in which the parasite does not undergo any development. However, in many cases, a paratenic host is essential for the transmission of the parasite and acts as a trophic bridge between the intermediate and definitive host (Baer, 1951). For example, some species of trematodes found as adults in the Eustachian tubes of frogs, use frogs as definitive hosts and aquatic microcrustaceans as intermediate hosts in their life cycles. However, because frogs do not generally consume microcrustaceans, a paratenic host must be involved in bridging the gap in trophic transmission. In this case, aquatic insects that commonly feed on microcrustaceans, such as damselflies and dragonflies, accumulate large numbers of these trematodes in their digestive tracts, and the trematodes do not develop in the microcrustacean. Frogs then eat the damselfly and dragonfly paratenic hosts and, in the process, become heavily infected (Bolek et al., 2010; Stigge and Bolek, 2015).
Most parasites must complete at least part of their life cycle by infecting 1 or more obligate, or required, hosts. In contrast, facultative parasites are usually not parasitic, but become so, opportunistically, when they encounter a potential host. For example, when certain species of free-living amoebas, such as Naegleria fowleri or species of free-living nematodes in the genus Halicephalobus are accidentally ingested or enter an opening of a novel host, they can establish within the host, and in some case cause serious and many times fatal conditions (Anderson et al., 1998; Kinde et al., 2000; Visvesvara et al., 2007). Similarly, when an obligate parasite infects a host which is different from its normal host, that host is called an accidental or incidental host. A number of cases have been reported of humans serving as accidental hosts for the nematode Angiostrongylus cantonensis, a species that normally resides in the lungs of various species of rats. Humans become infected with A. cantonensis by ingesting terrestrial gastropod intermediate hosts that are living on raw vegetables, such as lettuce (Pien and Pien, 1999). In humans, the nematodes migrate to the brain where they cause abscesses, brain swellings, and hemorrhages. Eventually, the juvenile nematodes die and degenerate. In this situation, humans can also be considered a dead-end host for A. cantonensis, because the parasite is not transmitted to functional hosts to continue its life cycle (Pien and Pien, 1999).
It should be noted that most, if not all, free-living species on our planet serve as hosts for many species of parasites. As a result, those free-living animals can serve different roles in the life cycles of different parasite species. One group of free-living animals that commonly serve as intermediate or paratenic hosts for numerous species of parasites are the gastropods (phylum Mollusca: class Gastropoda). Terrestrial, freshwater, and marine snails have been reported as intermediate and/or paratenic hosts for most species of digenetic trematodes, as well as various species of nematodes, tapeworms, and even acanthocephalans (Hopp, 1954; Dollfus, 1974; Rysavý, 1986; Lockyer et al., 2004; Lu et al., 2018). As an example, a single species of freshwater snail, Physa acuta, collected from various streams and wetlands across north-central Oklahoma, United States, serves as the first or second intermediate host for at least 9 species of flukes, and as a paratenic or accidental host for 3 species of horsehair worms, 1 species of nematode, and 1 species of thorny-headed worms, all of which infect various insects or vertebrates as definitive hosts (Gustafson and Bolek, 2016; Harkins et al., 2016; Koch, 2018; Figure 1).
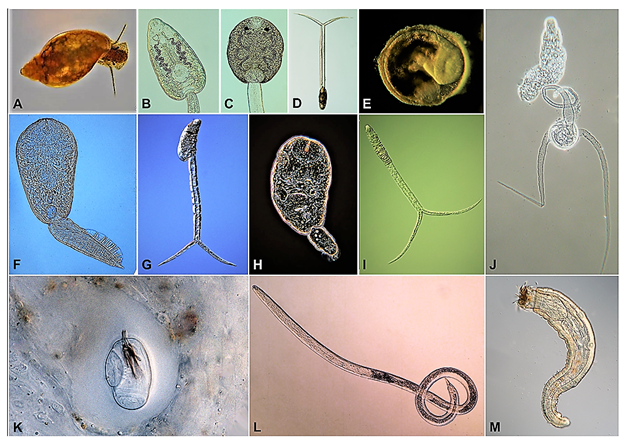
Figure 1. An example of a common North American freshwater snail, Physa acuta (A) and 12 species of parasites from 4 phyla representing different types of host associations. B–D, F–J) Show the cercarial stages of 8 species of digenetic trematodes which develop within the snail host and are released into the water column, to infect a second intermediate host. Physa acuta serves as the first intermediate host in the life cycles of these parasites. E) A metacercarial stage of the digenetic trematode Allassostomoides parvus which is infective to turtle definitive hosts. Physa acuta serves as the second intermediate host in the life cycle of this parasite. K) A cyst of a horsehair worm, Paragoridus varius in the tissue of P. acuta. Horsehair worms infect crickets and other arthropods as definitive hosts and they can use aquatic insects as paratenic hosts. Because crickets do not usually feed on aquatic snails, Physa acuta is considered an accidental host for this parasite. L–M) A juvenile Spiroxys contortus (a nematode) and a juvenile Neoechinorhynchus emydis (an acanthocephalan). Both of these parasites use microcrustaceans as first intermediate hosts and aquatic turtles as definitive hosts. Physa acuta may act as a an accidental/paratenic host for these parasites when individuals ingest infected microcrustacean first intermediate hosts and which are then eaten by the turtle definitive host.
(Source: M. Bolek. License: CC BY-NC-SA 4.0.)
Reservoir Hosts and Vectors
Another definition commonly used in the parasitology literature is the concept of reservoir host. Broadly defined, a reservoir species maintains a parasite infection in nature and serves as a source of infection for other species of animals. From a medical perspective, the definition of a reservoir host is usually restricted to any animal that maintains parasites as a source of infection for humans or domestic animals. In addition, many parasites that infect humans, domestic animals, and wildlife are transmitted by biological vectors. The term vector has been applied to a diverse group of potential animal hosts, and when used broadly in parasitology, can include any animal that transmits parasites from one host to another (Wilson et al., 2017). However, from a medical, ecological, and evolutionary perspective, a vector is defined as a mobile micropredator (for example, mosquito, leech, or vampire bat) that feeds on the blood or other bodily fluids of vertebrates and in some cases invertebrates (Figure 2). (Lafferty and Kuris, 2002; Wilson et al., 2017).
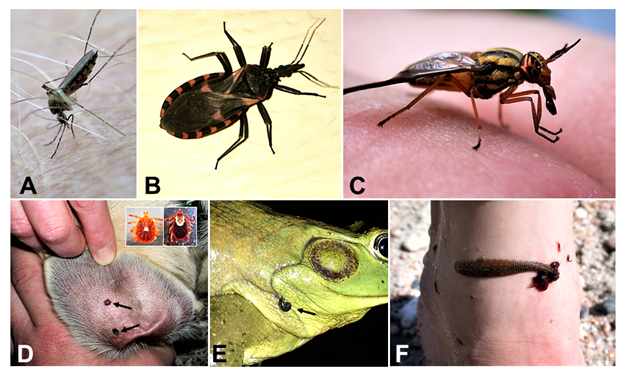
Figure 2. Examples of typical vector hosts. A) Female mosquito in the genus Aedes in the process of taking a blood meal. Note the specialized sucking mouth part injected into the skin of author M. Bolek. B) A reduviid bug. This is one of the primary biological vectors of Trypanosoma cruzi, the causative agent of Chagas disease. This species of bug can transmit the infective parasite stage to the vertebrate host through its feces. C) A female striped-backed deer fly Chrysops vittatus. Because of their blood feeding habits, many species of deer flies serve as mechanical vectors for parasites. Note the complex mouth parts, used to slice open the skin of the victim, after which the fly sips blood from the pooling blood on the surface of the skin. D) Females of 2 species of hard ticks, Amblyomma americanum and Dermacentor variabilis (arrows), attached and feeding on the ear of a stray dog Canis lupus familiaris. Ticks are common biological vectors for various parasites including protozoa and various helminths. E–F) Leeches (order Rhynchobdellida: family Glossiphoniidae) Placobdella picta (arrow) and P. rugosa feeding on a bullfrog Lithobates catesbeianus and the leg of Melissa Bolek (order Primates: family Hominidae), respectively. Leeches are common biological vectors for protozoan parasites of amphibians and reptiles. Note, in F the numerous young leeches feeding from the same bite wound as the mother leech.
(Source: M. Bolek. Informed consent obtained from the human subjects. License: CC BY-NC-SA 4.0.)
In most sanguinivorous species of animals that can also act as vectors of parasites, blood and/or tissue parasites from an infected animal may be ingested in 2 main ways; 1) Through telmophagy, in which the ectoparasitic animal abrades the skin and capillary beds of a vertebrate and a small hemorrhage forms, from which the animal vector then feeds, and 2) via solenophagy, in which a vector directly pierces blood vessels of its host to feed. For example, female horse flies and deer flies use telmophagy and when they feed, they lacerate the skin of their host with specialized cutting bladelike maxillae and then suck up the blood with sponge-like labellae (Matheson, 1950). In contrast, female mosquitoes are solenophagic feeders with mouthparts that are adapted to piercing vertebrate skin with their cutting maxillae and then suck blood with the hypopharynx (Choo et al., 2015; Mullen and Durden, 2009). Note that males do the same thing but with plants.
Based on their relationship with the parasite, vector-hosts can be assigned to 2 groups, including either mechanical or biological vectors. Mechanical vectors merely transmit the parasite between and among vertebrates, but without any multiplication or development of the parasite within the vector-host. Although not necessary for the multiplication or development of the parasite, mechanical vectors are essential for the transmission of various parasite species among its vertebrate hosts. A typical example includes flies (order Diptera) of the family Tabanidae (horse flies and deer flies) which are mechanical vectors for Trypanosoma evansi (order Kinetoplastida: Trypanosomatidae) of horses and other vertebrates (Bowman, 2013). Because female tabanids are not subtle and may cause pain when they bite their victim, they are usually quickly dislodged by defensive movements of the host and rarely remain on a host long enough to become fully engorged with blood. Instead, the tabanid quickly flies off the infected host and lands on another animal to feed again. In essence, it ingests blood frequently from multiple hosts and, in the process, it can mechanically and rapidly transmit T. evansi from one horse to another. In contrast to mechanical vectors, a biological vector is one in which the parasite multiplies and/or develops within organs and/or tissues of the vector host. Often, there is a time lag between acquisition of the parasite by the biological vector and the ability of the parasite to be transmitted by that vector to a new definitive host. This has been called the extrinsic incubation period.
Within biological vectors, and during the extrinsic incubation period, 3 types of multiplication and/or developmental patterns of the parasite can occur (Figure 3). Propagative transmission, involves simple amplification of a parasite within the vector-host. In this case, the same form of the parasite taken up by the vector multiplies within the vector and is then transmitted to a new vertebrate host. Examples include various species of bacteria, and some trypanosomatid protozoans, where the parasite multiplies within the vector-host but does not change morphologically. In contrast, cyclopropagative transmission, involves asexual and/or sexual multiplication of the parasite, and hence amplification of the parasite within the vector-host. Importantly, in cyclopropagative transmission, the form of the parasite transmitted to the next vertebrate host is morphologically distinct from the initial form taken up by the vector-host.
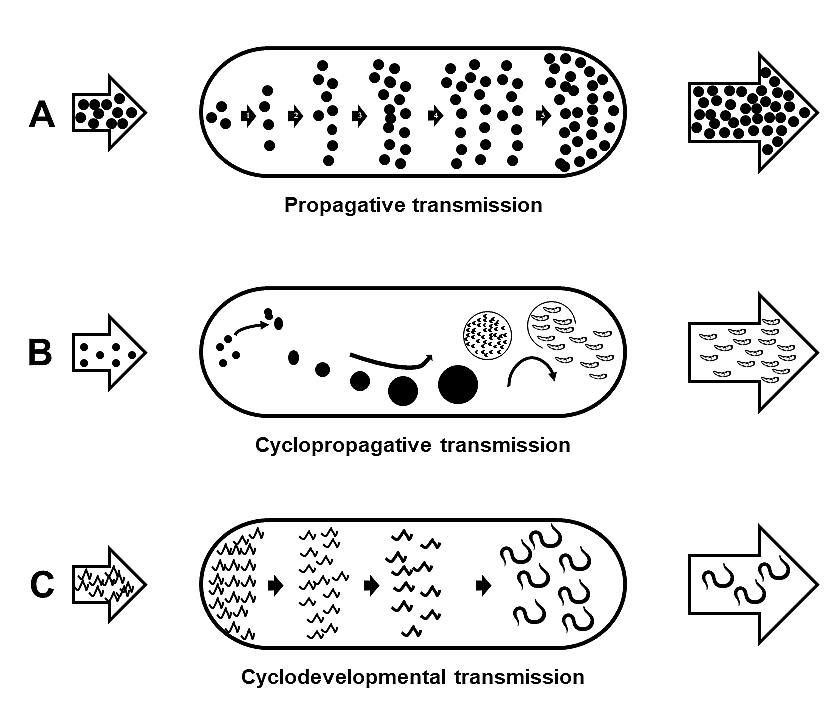
Figure 3. Types of biological associations between parasites and their vector hosts, represented by ovals. The arrows on the left indicate blood ingested by the vector from an infected vertebrate host, and the arrows on the right represent the infective parasite stage transmitted to another vertebrate host after a sufficient incubation period. A) Propagative transmission, the parasite multiplies within the vector, usually by an indefinite number of generations of binary fission. The stages transmitted are the same but far more numerous than originally acquired during the vector’s original blood meal. Examples of parasites with propagative transmission include some species of trypanosomatid protozoans. B) Cyclopropagative transmission, the parasite undergoes 1 or more cycles of asexual and/or sexual reproduction where it increases in numbers. The infective stage to the vertebrate host, is morphologically distinct from the form originally acquired during the vector’s original blood meal. Examples of parasites with cyclopropagative transmission include the causative agents of malaria and Chagas disease in humans. C) Cyclodevelopmental transmission, the parasite develops from the stage acquired by the vector host to an infective stage to the next vertebrate host, without any multiplication or reproduction. There is usually a loss of parasites from the original number acquired by the vector, and the final number that develop to the infective stage to the next host. Common examples of cyclodevelopmental parasites include filarioid nematodes.
(Source: Adapted from McClelland (1992), 2019. License: CC BY-NC-SA 4.0.)
An example of asexual cyclopropagative development occurs in the trypanosomatid Trypansoma cruzi within its reduviid bug vector-host; sequential cycles of asexual and sexual reproduction within mosquito and tick vector-hosts occur in various genera of apicomplexans such as Plasmodium and Babesia, respectively. As a result—and depending on the specific vector-host and parasite reproductive relationship within the vector—some biological vectors can be classified as definitive or intermediate hosts. In the case of Plasmodium in vertebrates and their mosquito host, the vertebrate is the intermediate host, while the mosquito is the definitive host because sexual reproduction occurs in the stomach wall of the mosquito. Finally, cyclodevelopmental transmission involves no multiplication of the parasite, but instead, the parasite develops within the vector to the next stage which is infective to the vertebrate host.
In cyclodevelopmental transmission, there is usually mortality and reduction in the number of parasites that are initially ingested by the vector relative to the number that are available when transmitted to the vertebrate host. Hence there is no amplification of the parasite in vector-hosts with cyclodevelopmental transmission. Examples of vector-borne parasites with cyclodevelopmental transmission include filarioid nematodes such as Litomosoides spp. (superfamily Filarioidea: family Onchocercidae), which depending on the particular species, reside in various tissues of vertebrate definitive hosts and release infected stages known as microfilariae into the blood, connective tissues, or skin. Once ingested by their mosquito intermediate vector-host the microfilariae develop to the next stage that is infective to the vertebrate host (Anderson, 2000).
Any parasites within the body of a vector-host must eventually exit the vector to be transmitted to a new host. Many vectors transmit parasites between successive vertebrate hosts during blood feeding. In some mechanical vectors, the parasites may be regurgitated back into the mouthparts and subsequently transmitted to a new vertebrate host during a blood feeding session. Similarly, in many biological vectors, the parasite is transmitted to a vertebrate host through inoculation or contaminated mouthparts during blood feeding. It is important to note, however, that not all vector-hosts transmit parasites between successive vertebrate hosts while taking a blood meal. This is particularly true for parasites that develop to the infective stage within the hindgut, or in the hemocoel of their vector-hosts and, as a result, cannot be transmitted through inoculation via contaminated mouthparts (Figure 2). The causative agent of Chagas disease Trypanosoma cruzi is one such example. Trypanosoma cruzi protozoans develop to the infective stage in the hindgut of their kissing bug vector, which includes various species of kissing bugs, such as Triatoma sanguisuga, and is then transmitted to the vertebrate host in the feces, when the bug defecates while feeding. Humans become infected when they scratch the bite wound, rub their eyes, or move the feces of the bug into the mucus membranes of the mouth or nose. These actions inadvertently inoculate the infective stages of T. cruzi in the bug’s feces into the various infection portals. Similarly, the apicomplexan parasite Hepatozoon americanum infects dogs as the intermediate host, and the lone star tick, Amblyomma americanum, as the vector definitive host. In this case, the parasite develops to the infective stage in the hemocoel of the tick vector and dogs become infected when they ingest infected ticks while grooming (Ewing and Panciera, 2003).
Finally, species-specific interactions between parasites and the type of reservoir and vector-hosts they employ in their life cycles can become extremely convoluted. In some cases, both mechanical and biological vectors can transmit a single parasite species. As mentioned previously, Trypanosoma evansi is transmitted to horses through the bite of blood sucking flies Tabanus and Stomoxys which act as mechanical vectors across Asia and in North Africa (in addition to Glossina), where T. evansi is endemic. However, T. evansi has relatively recently been introduced into Central America and South America, where it can be transmitted to horses by one of the species of vampire bats, Desmodus rotundus, which can serve as both vector and reservoir host (Brun et al., 1998). Vampire bats become infected with T. evansi by feeding on the blood of infected horses. Parasites enter the bat’s bloodstream through the mucus membranes lining the buccal cavity, and some of the infected bats die due to disease caused by the initial phase of infection (Desquesnes et al., 2013). However, some individuals survive the initial infection with the trypanosomes achieving a chronic infection with high blood parasitemia and some individual bats with a chronic infection have trypanosomes in their saliva. These bats then act as biological vectors and can transmit T. evansi to horses via their saliva during blood feeding. Additionally, because infected vampire bats commonly groom each other and/or feed other bats in the colony regurgitated blood, these infected vampire bats can propagate the infection among other individals in the colony (Desquesnes et al., 2013). As a result, vampire bat colonies can maintain T. evansi in the absence of infections in horses, and the infected bats can serve as reservoir hosts for infections in horses! Finally, there are reports of canids becoming infected by eating freshly killed mammals that are infected with T. evansi (see Woo, 1977).
Literature Cited
Anderson, R. C. 2000. Nematode Parasites of Vertebrates: Their Development and Transmission, 2nd edition. CAB International, Wallingford, United Kingdom, 650 p.
Anderson, R. C., K. E. Linder, and A. S. Peregrine. 1998. Halicephalobus gingivalis (Stefanski, 1954) from a fatal infection in a horse in Ontario, Canada with comments on the validity of H. deletrix and a review of the genus. Parasite 5: 255–261. doi: 10.1051/parasite/1998053255
Baer, J. G. 1951. Ecology of Animal Parasites. University of Illinois Press, Urbana, Illinois, United States, 224 p.
Bolek, M. G., H. R. Tracy, and J. J. Janovy, Jr. 2010. The role of damselflies (Odonata: Zygoptera) as paratenic hosts in the transmission of Halipegus eccentricus (Digenea: Hemiuridae) to anurans. Journal of Parasitology 96: 724–735. doi: 10.1645/GE-2365.1
Bowman, D. D. 2013. Georgis’ Parasitology for Veterinarians, 10th edition. Saunders, Philadelphia, Pennsylvania, United States, 496 p.
Brun, R., H. Hecker, Z.-R. Lun. 1998. Trypanosoma evansi and T. equiperdum, distribution, biology, treatment, and phylogenetic relationship: A review. Veterinary Parasitology 79: 95–107. doi: 10.1016/S0304-4017(98)00146-0
Choo, Y. M., G. K. Buss, K. Tan, and W. S. Leal. 2015. Multitasking roles of mosquito labrum in oviposition and blood feeding. Frontiers in Physiology 29: 306. doi: 10.3389/fphys.2015.00306
Combes, C. 2005. The Art of Being a Parasite. University of Chicago Press, Chicago, Illinois, United States, 291 p.
Craig, S. F., L. B. Slobodkin, G. A. Wray, and C. H. Biermann. 1997. The ‘paradox’ of polyembryony: A review of the cases and a hypothesis for its evolution. Evolutionary Ecology 11: 127–143. doi: 10.1023/A:1018443714917
Desquesnes, M., A. Dargantes, D.-H. Lai, Z.-R. Lun, et al. 2013. Trypanosoma evansi and Surra: A review and perspectives on transmission, epidemiology and control, impact, and zoonotic aspects. Biomedical Research International 2013: 321237. doi: 10.1155/2013/321237
Dollfus, R.-P. 1974. Énumération des cestodes du plancton et des invertébrés marins, 8e contribution: Avec un appendice sur le genre Oncomegas R.-Ph. Dollfus 1929. Annales de Parasitologie humaine et comparée 49: 381–410. doi: 10.1051/parasite/1974494381
Euzet, L., and C. Combes. 1980. Les problèmes de l’espèce chez les animaux parasites. Bulletin de la Société Zoologique France 40: 239–285.
Ewing, S. A., and R. J. Panciera. 2003. American canine hepatozoonosis. Clinical Microbiology Reviews 16: 688–697. doi: 10.1128/CMR.16.4.688–697.2003
Gustafson, K. D., and M. G. Bolek. 2016. Effects of trematode parasitism on the shell morphology of snails from flow and nonflow environments. Journal of Morphology 277: 316–325. doi: 10.1002/jmor.20497
Harkins, C., R. Shannon, M. Papeş, A. Schmidt-Rhaesa, et al. 2016. Using gordiid cysts to discover the hidden diversity, potential distribution, and new species of gordiids (Phylum Nematomorpha). Zootaxa 4088: 515–530. doi: 10.11646/zootaxa.4088.4.3
Hopp, W. B. 1954. Studies on the morphology and life cycle of Neoechinorhynchus emydis (Leidy), an acanthocephalan parasite of the map turtle, Graptemys geographica (Le Sueur). Journal of Parasitology 40: 284–299. doi: 10.2307/3273740
Kinde, H., M. Mathews, L. Ash, and J. St. Leger. 2000. Halicephalobus gingivalis (H. deletrix) infection in two horses in southern California. Journal of Veterinary Diagnostic Investigations 12: 162–165. doi: 10.1177/104063870001200213
Koch, R. W. 2018. Distribution and interactions of turtle acanthocephalans in two species of freshwater snails. MS thesis–Oklahoma State University, Stillwater, Oklahoma, United States, 91 p.
Lafferty, K. D., and A. M. Kuris. 2002. Trophic strategies, animal diversity and body size. Trends in Ecology and Evolution 17: 507–513. doi: 10.1016/s0169-5347(02)02615-0
Lockyer, A. E., C. S. Jones, L. R. Noble, and D. Rollinson. 2004. Trematodes and snails: An intimate association. Canadian Journal of Zoology 82: 251–269. doi: 10.1139/z03-215
Lu, X.-T., Q.-Y. Gu, Y. Limpanont, L.-G. Song, et al. 2018. Snail-borne parasitic diseases: An update on global epidemiological distribution, transmission interruption and control methods. Infectious Diseases of Poverty 7: 28. doi: 10.1186/s40249-018-0414-7
Matheson, R. 1950. Medical Entomology, 2nd edition. Comstock Publishing, Ithaca, New York, United States, 612 p.
McClelland, G. A. H. 1992. Medical Entomology: An Ecological Perspective, 12th edition. University of California, Davis, Davis, California, United States, 332 p.
Mullen, G. R., and L. A. Durden. 2009. Medical and Veterinary Entomology, 2nd edition. Elsevier Academic Press, London, United Kingdom, 637 p.
Pien, F. D., and B. C. Pien. 1999. Angiostrongylus cantonensis eosinophilic meningitis. International Journal of Infectious Diseases 3: 161–163. doi: 10.1016/S1201-9712(99)90039-5
Rysavý, B. 1986. Water snails as paratenic hosts of Hymenolepididae Fuhrmann, 1907 in Czechoslovakia. Folia Parasitologica 33: 219–226.
Stigge, H. A., and M. G. Bolek. 2015. The alteration of life history traits and increased success of Halipegus eccentricus through the use of a paratenic host: A comparative study. Journal of Parasitology 101: 658–665. doi: 10.1645/15-793
Tinsley, R. C. 1990. Opportunism in parasite life cycles. In C. J. Barnard and J. M. Behnke, eds. Parasitism and Host Behavior, Burgess Science Press, London, United Kingdom, p. 158–192.
Visvesvara, G. S., H. Moura, and F. L. Schuster. 2007. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunology and Medical Microbiology 50: 1–26. doi: 10.1111/j.1574-695X.2007.00232.x
Wilson, A. J., E. R Morgan, M. Booth, R. Norman, et al. 2017. What is a vector? Philosophical Transactions of the Royal Society B 372: 20160085. doi: 10.1098/rstb.2016.0085
Woo, P. T. K. 1977. Salivarian trypanosomes producing disease in livestock outside of sub-Saharan Africa. In J. P. Kreier, ed. Parasitic Protozoa. Academic Press, New York, New York, United States, p. 269–296.
Noun
From Latin: hospes = guest or host
Definition: Any living organism in or on which a parasite lives and/or feeds
Definition: Host in which the terminal (frequently sexual) stage of the parasite occurs
Synonym: Primary host
Definition: One which alternates with the definitive host in which the parasite passes through partial development, but not to sexual maturity
Noun
Adjective: polyembryonic
From Greek: polys = many; embryon = fetus
Definition: The formation of multiple embryos from a single egg
Definition: A host harboring a parasite that does not undergo further development and is generally of ecologic advantage in the disease cycle
Definition: A parasite that cannot exist without a host during all or some portion of the life cycle. see facultative parasite
Definition 1: A parasitic organism that can develop inside a host, but still retains the ability to complete a free-living life cycle in the outside environment
Definition 2: Organisms normally free-living that may become parasitic under special environmental conditions
Definition: A host in which a pathogenic parasite is not commonly found
Definition: A definitive host in which the infection usually resides in nature
Noun
From Latin: vehere = to carry
Definition 1: Any carrier, particularly an animal, that transmits a disease organism from one host to another
Defintion 2: In helminthic disease, an intermediate host that seeks out the definitive host; such as a mosquito
Adjective
From Latin: sanguis = blood; vorare = to devour
Definition: Feeding on blood
Noun
From Greek: telma = pool; phagein = to eat
Definition: An arthropod that severs the skin and blood vessels of a host animal, causing a small blood hemorrhage so as to feed
Noun
From Greek: solen = pipe; phagein = to feed
Definition: A blood-feeding arthropod whose mouthparts pierce directly into a host's blood vessel to feed
Noun
From Latin: trans = across; mittere = to send
Definition 1: Horizontal: the transfer of an infectious agent from one organism to another
Definition 2: Vertical: transmission from one generation to another

