5 Life Cycles
Matthew G. Bolek; Kyle D. Gustafson; and Gabriel J. Langford
Introduction
Life cycles of parasites have evolved into complex sequences of improbable events, with as many as 4 host species being included in the life cycle of certain parasite species (Bolek et al., 2010). Relative to their hosts, parasites in their infective stages are rather small and have limited mobility in the external environment. As a result, one can make the argument that the most dangerous part of any parasite’s life cycle is when the parasite is away from its host. Consequentially, adaptive scenarios and evolutionary contingencies are both often invoked to explain the complexity of parasite life cycles and the resulting transmission events (Poulin and Cribb, 2002).
In order for a particular parasite to infect and live in or on an appropriate host, there must be suitable conditions en-abling access to the host(s), including: 1) A dependable means of transmission from one host to another, 2) the ability of the parasite to establish itself within that host after reaching it, and 3) specific conditions within that host for the parasite to survive, grow, and reproduce. To accomplish this, parasites have evolved various types of life cycles which enable them to complete the necessary steps (that is, colonize, survive, grow, and mature) among a variety of different but often specific host species. A parasite life cycle is defined broadly as including the ontogenetic stages of a specific parasite species, and a set of events, such as growth and reproduction, that must occur before the parasite can survive and reproduce. In the case of parasites, the life cycle also includes all necessary hosts and all transmission events that enable a specific species of parasite to complete its life cycle.
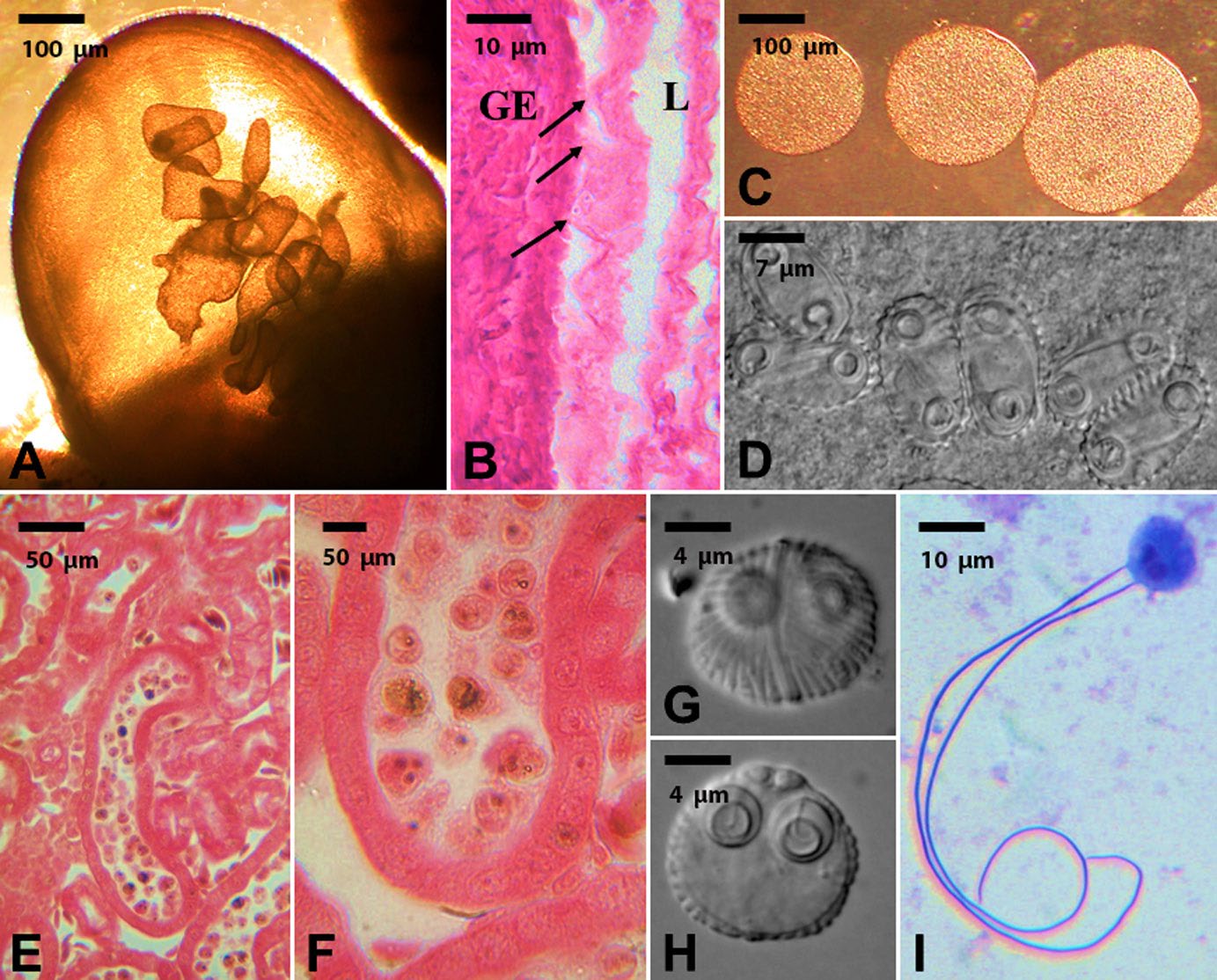
Figure 1. Example of coelozoic parasites with restricted site specificity; showing detailed development of the next infective stage in the life cycle. A–D) Developmental stages of Cystodiscus serotinum in the gallbladder of a green frog Rana clamitans. A) Gallbladder showing developing plasmodia stages. Scale bar = 100 μm. B) Histological section showing the distribution of plasmodia in the lumen (L) of the gallbladder and their intimate association (arrows) with the epithelial cells of the gallbladder (GE). Scale bar = 10 μm. C) Removed plasmodia from the gallbladder. Scale bar = 100 μm. D) Infective spore stages within the plasmodia. Scale bar = 7 μm. E–F) Developmental stages of Sphaerospora ohlmacheri in the kidneys of a Blanchard’s cricket frog Acris blanchardi. E) Histological section of the kidney showing renal tubule occluded by plasmodia of Sphaerospora ohlmacheri. Scale bar = 50 μm. F) Close up of renal tubule occluded with developing spores of Sphaerospora ohlmacheri. Scale bar = 50 μm. G–I) Detailed morphology of infective spores of Sphaerospora ohlmacheri. Note the detailed surface structures on the spores and the everted extruded polar filaments (I) indicating the spore stages are infective to the next host in the life cycle. Scale bars = 4 and 10 μm.
(Source: M. Bolek. License: CC BY-NC-SA 4.0.)
Infection Site
Depending on the species, many parasites occupy a specific infection site and/or location in 1 or more of the hosts infected during their life cycle. Parasites that inhabit the lumen of the intestines, lungs, or other hollow organs of their hosts are said to be coelozoic, whereas parasites that live within tissues of their hosts are referred to as histozoic. For example, amphibians are commonly infected with 2 distinct genera of myxozoans, a group of parasitic cnidarians (Jirků et al., 2006; 2007; Hartigan et al., 2012). Cystodiscus serotinum produces infective spore stages in the gallbladder of amphibians; whereas Sphaerospora ohlmacheri produces infective spores in the tubules of the kidneys of frogs and toads. Both species are coelozoic because they infect the lumen of the gallbladder or tubules of the kidneys. However, each species is considered site specific in amphibians, such that C. serotinum can only develop in the gallbladder and S. ohlmacheri can only develop in the tubules of the kidneys (Figure 1). In contrast, many cercaria, which are the larval stages of trematodes, are histozoic and encyst within various tissues of their second intermediate hosts. For example, tadpoles (larvae) of many amphibian species serve as second intermediate hosts for various trematode species (Rhoden and Bolek, 2015). The metacercariae of some trematode species only encyst in specific tissues and organs whereas metacercariae of other species are infection site generalists and can be found in various tissues and organs of tadpoles (Figure 2); thus, some species of trematodes have metacercariae that are generalists and some that are specialists. Studies indicate that cercariae of echinostomes actively seek and enter tadpoles via the cloaca, then migrate to the kidneys where they encyst (Thiemann and Wassersug, 2000; Taylor et al., 2004). In contrast, species of Telorchis will penetrate any surface on the body of a tadpole (Schell, 1962). Notably, tadpoles have a greater chance of becoming infected with species of Telorchis by mechanically sucking in infective stages of flukes from the water column, whereas cercariae of echinostomatid flukes can only infect tadpoles when they enter through the cloaca (Rhoden and Bolek, 2012).
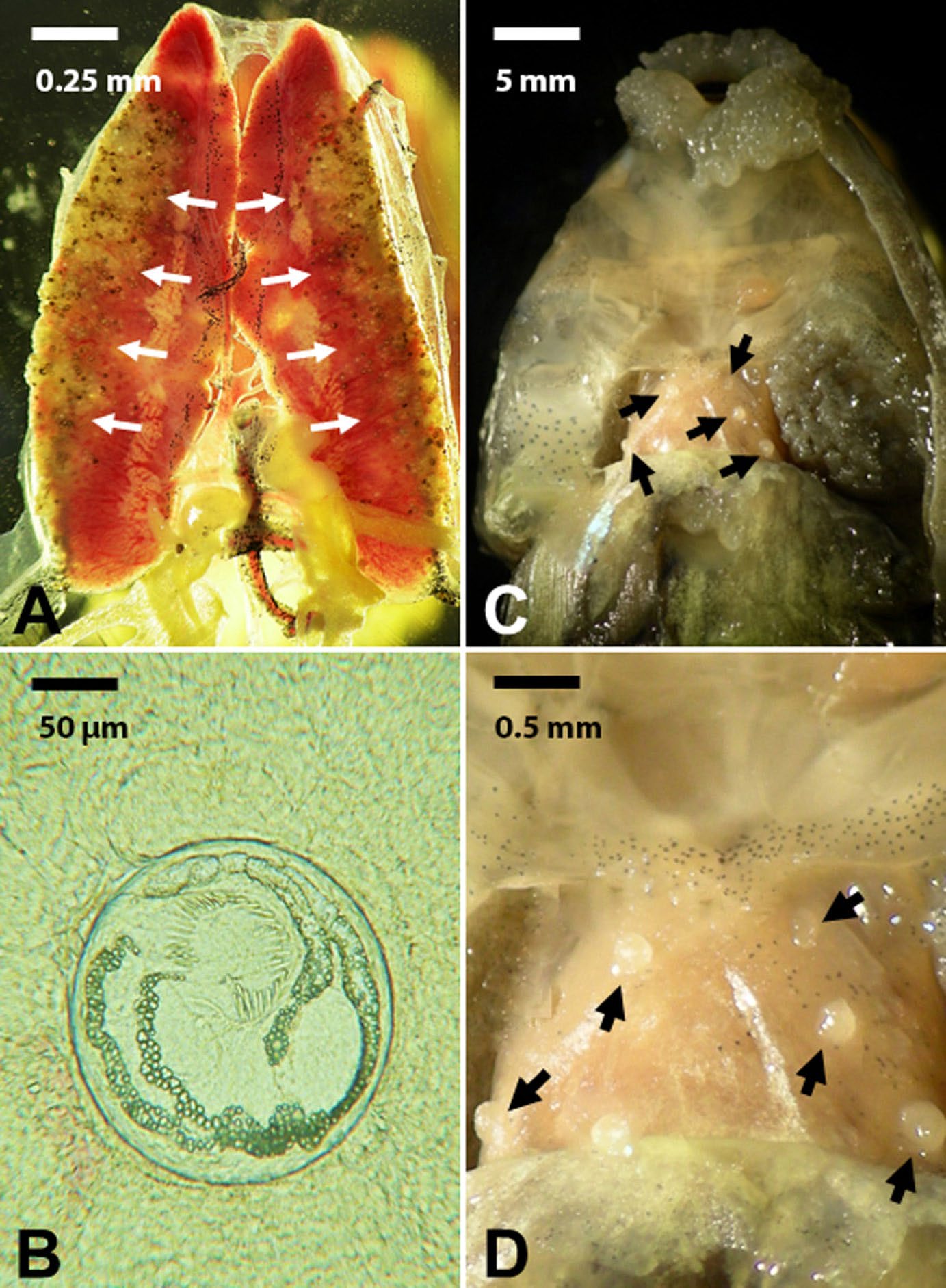
Figure 2. Example of histozoic parasites with variable site specificity in a bullfrog tadpole. A) Removed kidneys from a bullfrog tadpole showing hundreds of echinostomatid metacercarial stages encysted on the lateral sides of each kidney (arrows). Scale bar = 0.25 mm. B) A single echinostomatid metacercaria encysted in kidney tissue of bullfrog. Scale bar = 50 μm. C) Ventral body region of a bullfrog tadpole with the musculature removed showing encysted metacercarial stages of Telorchis sp. (arrows). Scale bar = 5 mm. D) Higher magnification of the heart showing the distribution of encysted metacercariae of Telorchis sp. (arrows) on the heart. Scale bar = 0.5 mm.
(Source: M. Bolek. License: CC BY-NC-SA 4.0.)
To a parasite, a host represents multiple microenvironments, and only certain environments meet the parasite’s very specific needs. Clearly, not all hosts will be equal, and some parasites that infect different host species can behave differently. As a result, the site of infection for some parasites can be influenced by their host species. For example, a recent study on the frog tongue fluke Halipegus occidualis showed that these flukes commonly infect 3 species of frogs (Stigge and Bolek, 2016). Anurans (frogs) become infected with H. occidualis when they ingest a dragonfly paratenic host that contains encysted metacercariae. However, when green frogs Lithobates clamitans and leopard frogs L. pipiens ingest an infected dragonfly paratenic host, the worms migrate from the stomach and attach to the lingual vein under the tongue, where they mate and lay eggs. In contrast, when dragonfly paratenic hosts are ingested by bullfrogs L. catesbeianus, the worms never attach to the lingual veins under the tongue, but instead reside in the frog’s stomach where they mate and lay eggs. It is unclear why H. occidualis behaves so differently in bullfrogs than in green frogs and leopard frogs; nonetheless, the study clearly indicates that different species of host-parasite combinations matter in understanding parasite life cycles and host-parasite interactions.
Host Specificity
To begin, note that host specificity is covered briefly in the introduction to this book. While there is some debate about whether this is the proper framework to consider life cycles, this contention will be left aside for now, since it is referenced extensively in the literature as it has served as the basis for numerous robust studies in the concept of ecology of parasites.
As observed with infection site, parasites can vary in their host range (also known as specificity) at 1 or more stages in their life cycles. Although most species of parasites are known to develop only in a restricted range of hosts, different parasites exhibit varying degrees of host specificity. For example, some species of cestodes, such as the pork tapeworm Taenia solium (Cyclophyllidea: Taeniidae) are only known to mature to egg producing adults in humans Homo sapiens definitive hosts and are considered host-specific at the definitive host level. In contrast, and at the other extreme, species of Trichinella (class Nemata: family Trichinellidae) can mature in almost any species of mammal. Another example of a parasite with a wide host range is the coccidian protozoan Toxoplasma gondii. This parasite uses cats (order Carnivora: family Felidae) as the definitive host (any cat species will do) but it can use almost any vertebrate as the intermediate host.
These examples exhibit the variety of host range shown by parasites across 3 phyla of phylogenetically unrelated parasites. A parasite that is specific for a single host species is said to be oioxenous, a parasite that infects closely related hosts is considered stenoxenous, whereas a parasite that infects unrelated hosts is considered euryxenous. Finally, some parasites exhibit stadium specificity where hosts are only susceptible to infection by a particular parasite at a specific developmental stage. Some protozoans such as gregarines (apicomplexans) that infect holometabolous insects and some species of nematodes and acanthocephalans that occur in amphibian definitive hosts, can only infect either the larva or adult stage of their host (Nickol and Heard, 1973; Clopton et al., 1992; Rhoden and Bolek, 2011; Childress et al., 2017). For example, tadpole pinworms, Gyrinicola batrachiensis are constrained to the large intestine of tadpole stages of anurans (Adamson, 1981). One explanation for this dramatic difference in host specificity between tadpoles and frogs is differences in their diets and digestive tracts. In general, pinworms feed on the bacteria found in the hindgut of animals that consume plants as a significant portion of their diet. As tadpoles metamorphose to the adult anuran stages, their feeding and correlated digestive tract changes dramatically from a predominantly herbivorous diet to a strictly carnivorous diet, and all G. batrachiensis are lost from their intestines (Adamson, 1981). As a result, separate and distinct parasite niches corresponding to distinct life cycle stages of free-living animals can affect parasite host range and measured specificity.
To understand the nature of host range, some parasitologists contend that experimental cross infections should be conducted to determine whether host-parasite associations may be established by true host-parasite incompatibility (Janovy et al., 2007). However, potential host species may simply not be infected with a particular parasite species because they never encounter the infective stage of the parasite in nature due to various environmental factors. With most systems involving parasites of vertebrates, logistical burdens make studying cross infection very difficult, especially when the species are not routinely reared in captivity. There are a number of studies on protozoa, trematodes, nematodes, and annelids testing host compatibility in insect, amphibian, and reptile host-parasite systems (Bolek and Janovy, 2007a; 2007b; 2008; Janovy et al., 2007; Bolek et al., 2009; 2010; Langford and Janovy, 2009; 2013; Childress et al., 2017; Andrews et al., 2015; Stigge and Bolek, 2016). In general, what these studies suggest is that host specificity has a strong ecological component, such that many potential and competent hosts never come in contact with the infective stages of a particular parasite species in nature, undoubtedly affecting host-parasite patterns of associations. Additionally, these studies indicate that it is difficult to predict the range of compatible hosts a particular parasite can infect. For example, Langford and Janovy (2013) tested the host specificity of 7 species of lungworms which infect snakes and anuran definitive hosts. Their field studies and experimental infections indicated that both species of snake lungworms were generalist snake parasites, and in nature and the laboratory they could infect up to 5 species of snakes. However, their laboratory experiments also suggested that lizards can be infected under some environmental conditions. In contrast, lungworms from anurans were found not to infect salamanders or reptiles in nature or in the laboratory. Additionally, amphibian lungworm species ranged from being strictly host specific, infecting only 1 species of frog or toad, to relative generalists, able to infect multiple species of distantly related frog and toad species. Overall, these studies indicate that for many parasite species, host specificity or host range in nature appears to be limited by both ecological and physiological factors, which vary among parasite species and their hosts.
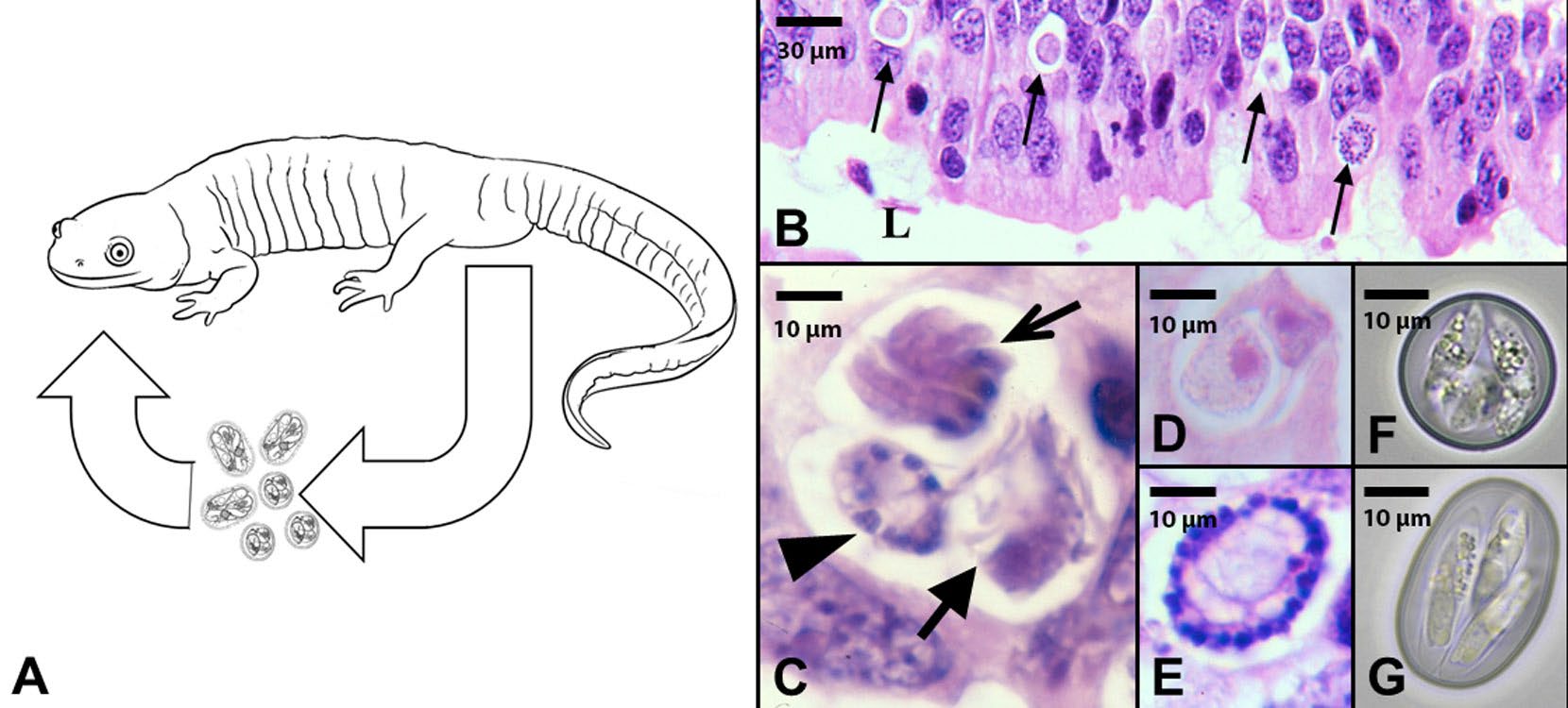
Figure 3. An example of a direct, monoxenous, but heterogenetic life cycle of salamander Eimeria spp. A) A tiger salamander Ambystoma tigrinum showing the routes of transmission of Eimeria species. Salamanders defecate infective stages (oocysts) into the external environment and become infected when they accidently ingest oocysts. B) Histological section of the small intestine of a tiger salamander showing different developmental stages of Eimeria species (arrows) in the epithelial cells of the small intestine. Scale bar = 30 μm. C) Higher magnification of an epithelial cell showing asexual multiplication (thin arrow) and development of microgametes (sperm; middle arrow) and macrogametes (ova; thick arrow). Scale bar = 10 μm. D–E) Epithelial cells showing developing oocysts (zygotes) after fertilization. Scale bar = 10 μm. F–G) Fully developed and infective oocysts recovered from the feces of Eimeria urodela and E. ambystomae. Scale bar = 10 μm.
(Source: M. Bolek. License: CC BY-NC-SA 4.0.)
Parasite Development and Types of Parasite Life Cycles
Parasite development can be categorized as monoxenous where the parasite lives and develops within a single host during its life cycle, or heteroxenous where a parasite lives and develops within more than 1 host during its life cycle. Additionally, life cycles can be categorized as simple or direct where a parasite only infects a single host in its life cycle, or as complex or indirect life cycles, where a parasite uses 2 or more hosts in its life cycle. However, some parasites with direct or indirect life cycles also go through complex reproductive events within their hosts where they alternate sexual and asexual generations in 1 or multiple hosts. As a result, distinct sets of terms are used to differentiate between parasite reproductive events within their hosts and life cycle complexity. For example, many coccidian species in the genus Eimeria have direct or simple life cycles and infect their definitive vertebrate host when the host ingests the infective oocyst stages. However, once inside the intestinal epithelial cells of its host, the coccidian goes through a complex set of multiple asexual multiplication events, followed by the production of male and female gametes and eventually sexual reproduction (Figure 3). Parasites that have alternations of sexual and asexual generations in their life cycle are commonly referred to as heterogenetic parasites. In contrast to Eimeria, all acanthocephalan species (phylum Acanthocephala) have indirect or complex life cycles, including a definitive, intermediate, and commonly an additional paratenic (transport) host. However, except for sexual reproduction in the definitive host, no other complex asexual multiplication or alternations of generations occurs in the intermediate or paratenic hosts in the life cycle (Figure 4). Parasites that have no alternation of sexual and asexual generations in their life cycles, are sometimes referred to as monogenetic parasites. As a result of the enormous diversity of parasite species, different combinations of direct or indirect and heterogenetic or monogenetic development can occur in different groups of parasites during their life cycles.
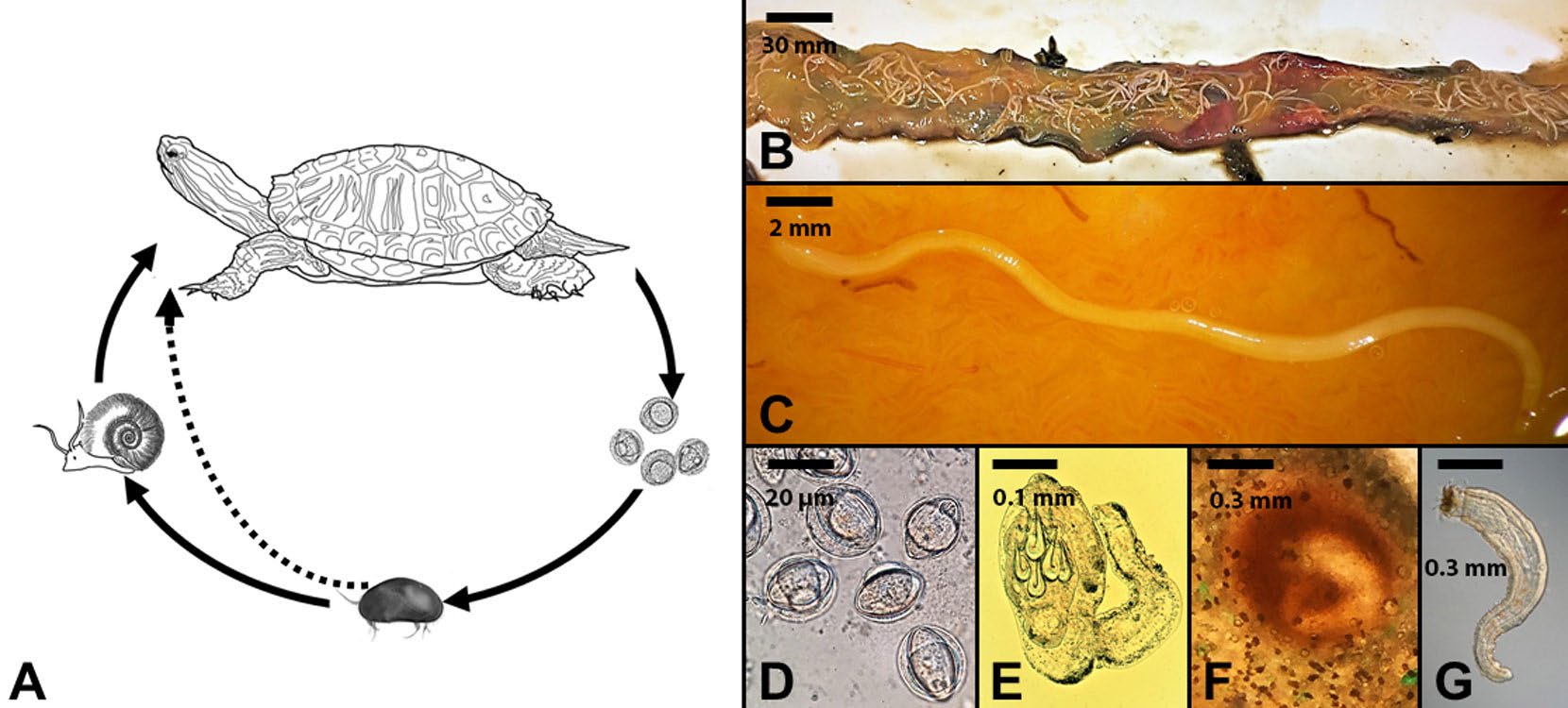
Figure 4. An example of an indirect, heteroxenous, but monogenetic life cycle of the turtle acanthocephalan Neoechinorhynchus emydis. A) Turtle definitive hosts release eggs into the external environment where they are ingested by ostracod intermediate hosts. Once the parasite develops to the next infective stages, the infected ostracod can be ingested by a snail paratenic host where no development of the parasite occurs or a turtle definitive host where sexual reproduction occurs. Additionally, turtles can become infected when they ingest snail paratenic hosts. B) The small intestine of a turtle showing hundreds of adult acanthocephalan parasites attached to the intestine. Scale bar = 30 mm. C) Higher magnification of a single adult female worm attached to the intestine mucosa. Scale bar = 2 mm. D) Eggs of an acanthocephalan. Scale bar = 20 μm. E) Developing larval stage recovered from the body cavity of an ostracod intermediate host. Scale bar = 0.1 mm. F) Encysted juvenile acanthocephalan in a snail paratenic host. Scale bar = 0.3 mm. G) Infected juvenile acanthocephalan removed from a snail paratenic host. Scale bar = 0.3 mm. Note the dramatic morphological changes among the different stages in the life cycle.
(Source: M. Bolek. License: CC BY-NC-SA 4.0.)
In addition to the examples above, there are other life cycle variations, particularly in parasite species that must exit their host into the external environment and develop into free-living adults and/or to find mates and reproduce in the external environment. For example, life cycles of some species of flies which cause myiasis (a term for an infestation of tissues, wounds, or body cavities of living animal by fly maggots) fall into this category (Zumpt, 1965). Many species of flies causing myiasis are obligate parasites and their maggots must develop within their hosts to complete the life cycle. For example, flies in the subgenus Bufolucilia commonly infect amphibian hosts throughout Europe and North America (Bolek and Coggins, 2002; Bolek and Janovy, 2004; Tantawi and Whitworth, 2014; Arias-Robledo et al., 2019). Female flies locate amphibian hosts visually and deposit eggs on the back and flanks of their unsuspecting frog or toad victims (Figure 5). The larvae hatch, migrate through the skin, and eventually disappear into the frog’s tissues. Within 2 to 3 days of infection, an open wound appears and displays the posterior spiracles of the maggots, which allows the maggots to breathe (Figure 5). Within these wounds, maggots develop to mature third instar larvae within 5 to 7 days of hatching, migrate out of the amphibian host, burrow into the soil, turn into pupae, metamorphose into adult flies, mate, and start the process all over again.
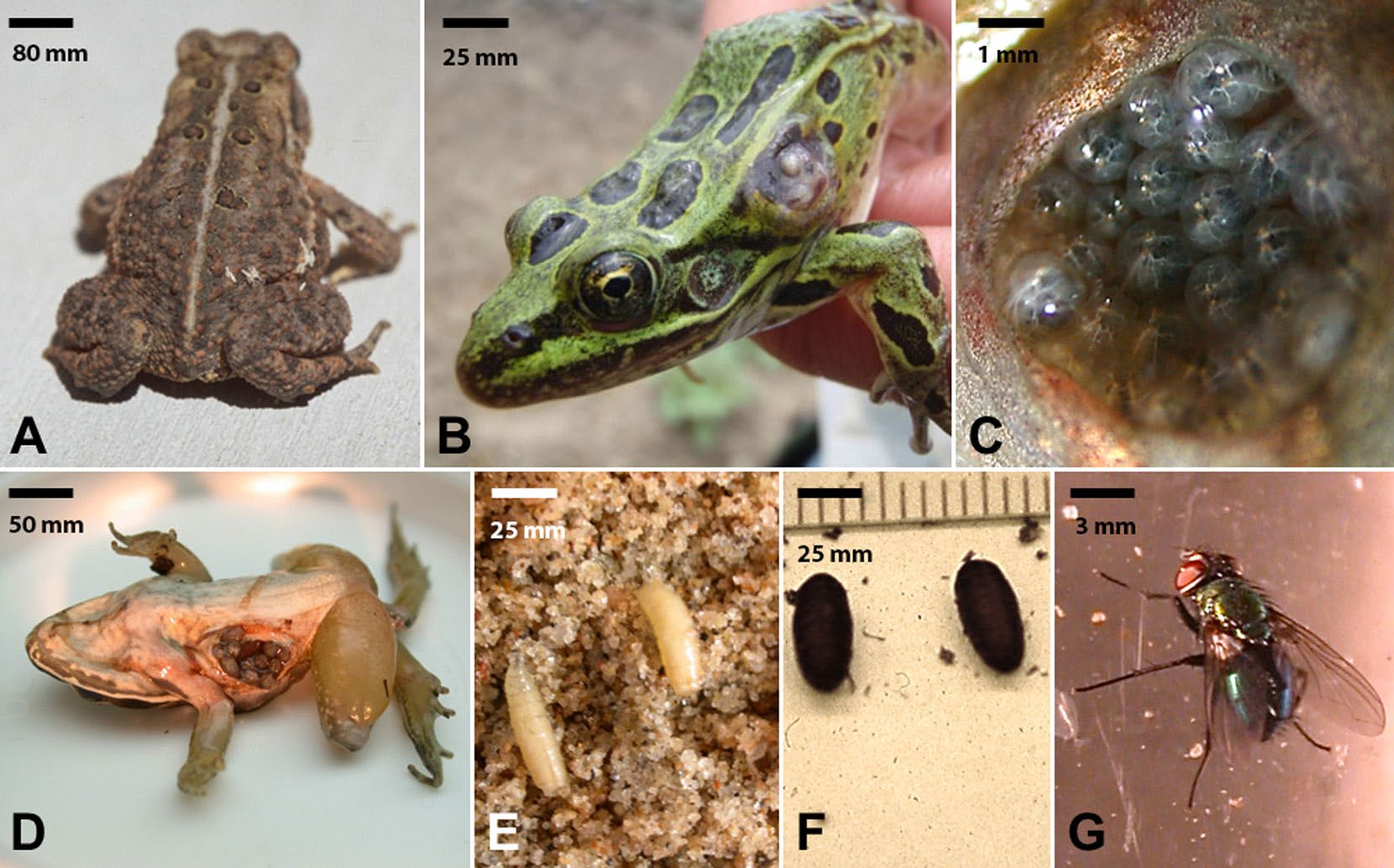
Figure 5. An example of a direct life cycle parasite where the parasites must exit the host and develop into a free-living adult and reproduce. A) Eggs of Bufolucilia silvarum glued to the back of an American toad Bufo americanus. Scale bar = 80 mm. B) Opened wound on the left lateral side of a northern leopard frog Rana pipiens. Note visible third instar maggots of Bufolucila silvarum in the wound. Scale bar = 25 mm. C) Third instar maggots of Bufolucila silvarum congregating and feeding as a group in an infected wood frog Rana sylvatica. Scale bar = 1 mm. D) Third instar maggots of Bufolucila elongata in a single wound on the right ventral side of a wood frog Rana sylvatica. Scale bar = 50 mm. E) Third instar maggots of Bufolucila silvarum searching for a place to pupate after leaving the host. Scale bar = 25 mm. F) Fully formed pupae of Bufolucila silvarum. Scale bar = 25 mm. G) An adult male green toad fly Bufolucila silvarum. Scale bar = 3 mm.
(Source: M. Bolek. License: CC BY-NC-SA 4.0.)
Other variations on parasite life cycles include the alternation of free-living and parasitic generations known as heterogonic reproduction. For example, lung nematodes in the genus Rhabdias alternate between parasitic and free-living generations. Parasitic individuals within the lungs of their amphibian hosts are protandrous hermaphrodites, a term for individuals that are functional males before becoming females. The spermatozoa are used to fertilize the eggs, and the eggs are then transported from the host’s lungs into the gastrointestinal tract, and defecated into the soil (Runey et al., 1978). The released eggs hatch and begin a free-living generation resulting in adult free-living males and females which undergo sexual reproduction in the external environment (Langford and Janovy, 2009). Next, the free-living female nematode’s progeny hatch within her body, where they feed on her internal organs, killing their mother in the process, and exiting her body as infective stages, a process known as matricidal endotoky. Finally, the infective juveniles enter the anuran host body cavity orally and/or via skin penetration and eventually migrate to the lungs to begin egg production to continue the life cycle (Baker, 1979).
The Role of Parasite Life Cycles in Transmission
Arguably, some of the most complex parasite life cycles belong to the digenetic trematodes, also known as flukes. During their life cycle, trematodes undergo sexual reproduction in the definitive host, followed by asexual reproduction in the first intermediate host in a process known as polyembryony, the formation of more than 1 embryo from a single fertilized ovum. Hundreds to thousands of free-living stages are then released from the first intermediate host, some of which infect a second intermediate host, which is then ingested by the definitive host (Figure 6).
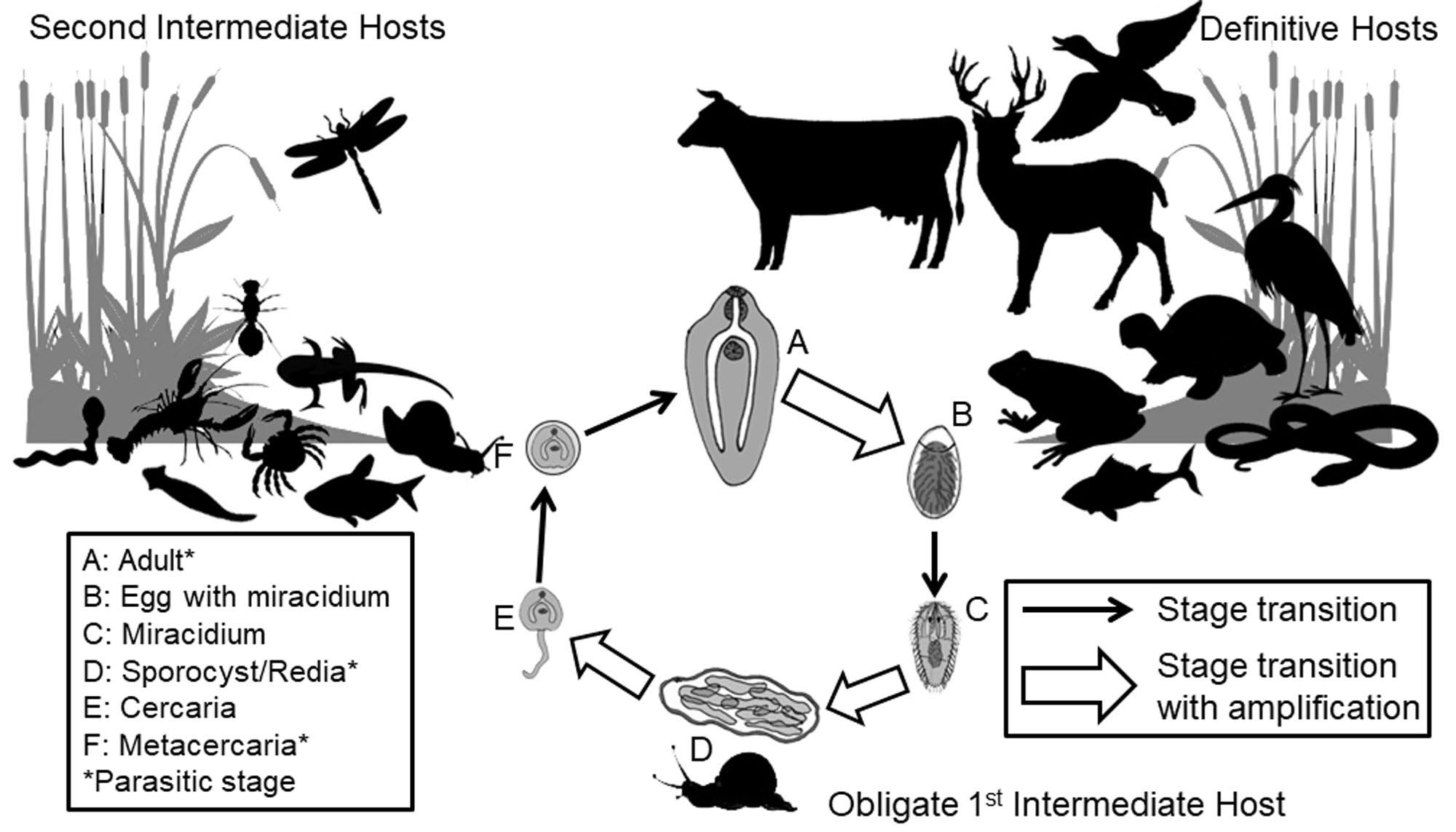
Figure 6. A representative diagram of a typical complex life cycle of a digenetic trematode. Note that most digenetic trematodes are host specific at the snail first intermediate host in the life cycle and much less host specific at the second intermediate and definitive host level. Also note the trematode developmental stages in the life cycle (A–F); including adult worms (A) producing eggs (B) through sexual reproduction in the definitive host (C), asexual reproduction (D) and production of free living cercariae (E) in the obligate snail first intermediate host.
(Source: M. Bolek. License: CC BY-NC-SA 4.0.)
A typical digenetic trematode life cycle offers a good example of the complexity of the transmission challenges faced by parasites during their life cycles (Lafferty and Kuris, 2002). First, eggs released into the external environment by adult worms in the definitive host hatch into a short-lived miracidium stage, which then must find a suitable first intermediate host, usually a snail. Second, and after asexual reproduction within the snail first intermediate host, the free-living but short lived cercariae emerge from the snail and must locate a suitable second intermediate host where they encyst as metacercariae stages. Third, the metacercaria stage must be ingested along with the second intermediate host by an appropriate definitive host for the life cycle to be completed.
It is hypothesized that parasites with complex life cycles have evolved by either adding or subtracting hosts based on trophic interactions of potential hosts (Poulin and Cribb, 2002). In trophically transmitted parasites with more than 1 host, or in parasites that are transmitted by vectors that take a blood meal from a vertebrate host, there are 2 hypotheses that support the addition of a host. One hypothesis proposes that the original host was preyed upon by other potential hosts higher up in the trophic food chain, and all other hosts have been added over time to the parasite life cycle (Smith-Trail, 1980; Poulin, 2007; Parker et al., 2003). Another hypothesis suggests the opposite. In this case, the original host was a top predator in the food web, and all other hosts with lower positions in the food web than the original host have been added secondarily to the parasite life cycle (Smith-Trail, 1980; Gibson and Bray, 1994; Lafferty, 1999; Parker et al., 2003). Finally, hosts can also be lost if the life cycle no longer requires a particular host for completion (Poulin and Cribb, 2002; Parker et al., 2003).
A number of studies on parasite life cycles indicate that some species of parasites can survive in the alimentary canal of the predators of their definitive hosts. For example, post-cyclic transmission has been reported in a number of acanthocephalan and nematode species (Bolek, 1997; Nickol, 2003). In these cases, when a predator ingests a definitive host, instead of dying or being lost, the parasites simply reattach themselves to the intestine of the predator and resume growth or reproduction. Importantly, the predator may be the same as or a different species than the original definitive host of the parasite. Additionally, the direct life cycle of aspidobothrean trematodes, which parasitize molluscs, commonly promotes their survival and they reproduce in the intestines of turtles and fish that in turn consume infected clams as part of their diet. The aspidobothrean trematodes are considered a basal sister group to the digenetic trematodes which also infect molluscs as first intermediate hosts, but have complex life cycles (Zamparo and Brooks, 2003). As a result, one can imagine the evolution of complex life cycles by the addition of hosts to a direct life cycle.
However, understanding the specific steps of how and why these life cycles have evolved is difficult to decipher due to the lack of a fossil record for most parasites, complex host-parasite associations, and the lack of empirical data on host use for most parasite species in nature (Stigge and Bolek, 2015). For example, it is currently unclear if these processes occur gradually or require less evolutionary time (Stigge and Bolek, 2015). As a result of these difficulties, understanding how life cycles operate in nature and what hosts are used by those parasites can provide empirical data for future hypotheses testing on parasite life cycle evolution.
Parasite Adaptations, and Life Cycle Variation and Plasticity
Reproduction is certainly the most important task that individuals of any species of parasite must accomplish during their lifespan within a definitive host. However, in order for any parasite to reproduce within its host, it must be able to infect that host. In combination, these 2 principles (infection and reproduction) dictate that parasite life cycles have been selected for their ability to increase the probability that individual propagules will infect their hosts and achieve reproductive output (consisting of more propagules).
Understanding parasite life cycles is fundamental for many types of parasitological inquiries because life cycles inform understanding of life history strategies, host-parasite interactions, community and population ecology, life cycle evolution, and the epidemiology of diseases. Yet, the propensity for biologists to portray life cycles as a fixed, invariable unit is a monumental error, as actual real-world life cycles are not captured fully in so-called iron wheel diagrams such as those depicted in textbooks or health agency websites (Bolek et al., 2016). Indeed, understanding life cycle plasticity and variability is crucial to understanding how parasites evolve and function in hosts and the external environment. Despite the importance of this area of investigation, few biologists focus on life cycles of parasites as the center of their research. Furthermore, most parasitologists who have studied life cycles only do so until the life cycle could be completed. Once elucidated, most investigators do not continue to search for alternative hosts to complete the life cycle in nature. It is therefore unsurprising that published life cycles tend to be accepted as absolute truth and their validity is rarely questioned (Krull, 1952; Bolek and Janovy, 2008; Bolek et al., 2009; 2010). Two examples are given in the following that provide realistic snapshots of how some parasites live in nature, while also highlighting specific life cycle adaptations that may increase both transmission probabilities and reproduction. In addition, these examples demonstrate how unrealistic paradigmatic life cycle diagrams are in deciphering transmission strategies of parasites in nature (Bolek et al., 2016).
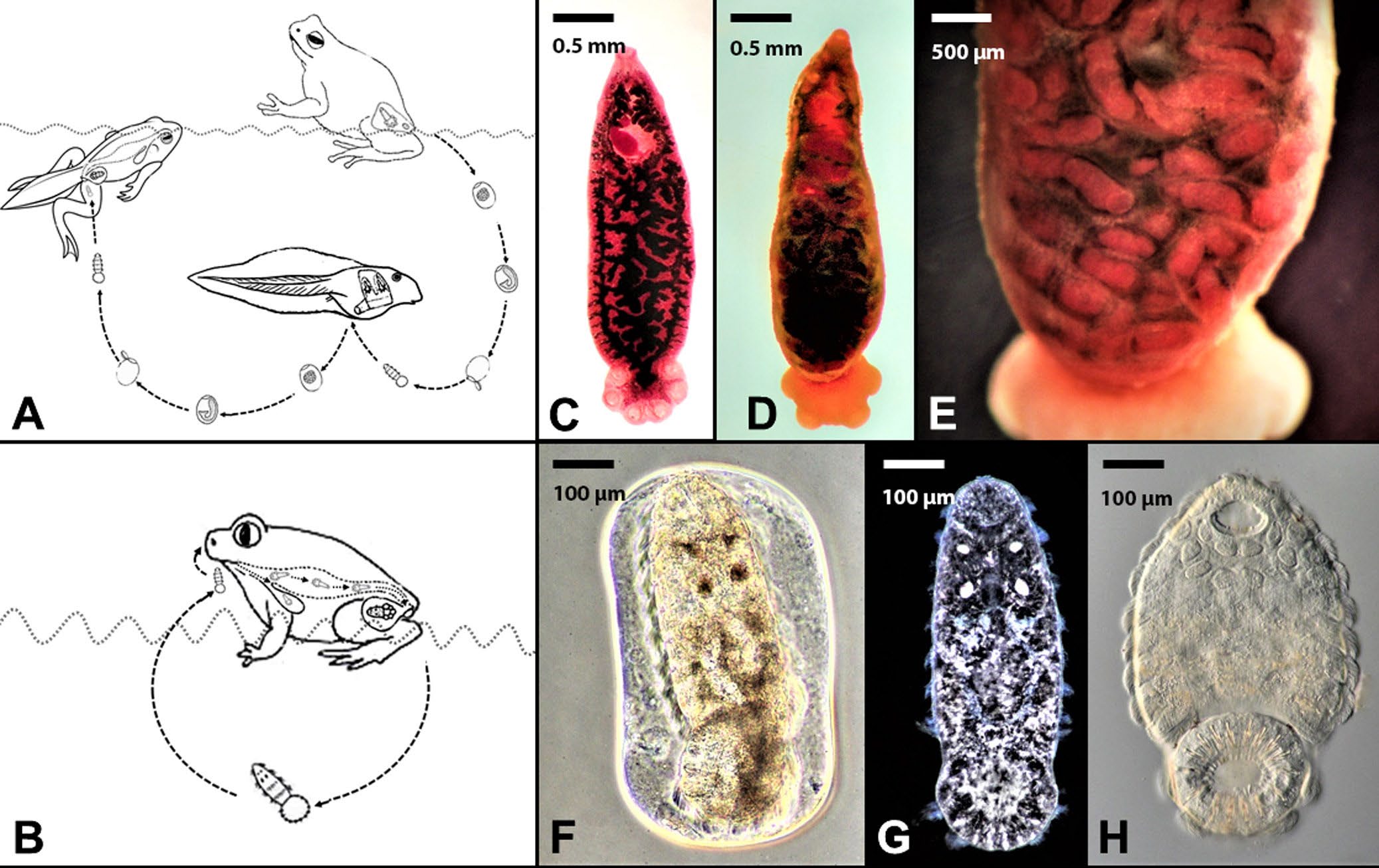
Figure 7. Example of life cycle variation for 2 closely related and host specific polystomatid trematodes. A) Transmission strategies of Polystoma nearcticum in the eastern gray treefrog Hyla versicolor. Note the egg being released by the urinary bladder generation of worms when their treefrog hosts enter ponds to breed followed by eggs being released from the branchial generation of worms on the gills of tadpoles. In all cases the eggs must develop in the external environment and the larval stage must find and infect metamorphosing froglets by entering their cloaca. B) Transmission strategy of Pseudodiplorchis americanus in Couch’s spadefoot toad, Scaphiopus couchii. Larval stages are released directly from the bladder of spadefoot toads when they enter breeding pools. C) Adult Po. nearcticum recovered from the urinary bladder of a Cope’s gray treefrog H. chrysoscelis. Scale bar = 0.5 mm. D) Adult Ps. americanus recovered from the urinary bladder of a Couch’s spadefoot toad S. couchii. Scale bar = 0.5 mm. E) Higher magnification of Ps. americanus showing fully developed larvae in the uterus. Scale bar = 500 μm. F–H) Egg and hatched larvae of Ps. americanus. Note the 4 eyespots in (F) and (G) and the ciliated cells containing hundreds of cilia used for swimming in (H). Scale bar = 100 μm.
(Source: M. Bolek. License: CC BY-NC-SA 4.0.)
The first example considers the life cycles of 2 closely related but host specific species of polystomatid flatworms (phylum Platyhelminthes: family Polystomatidae): Polystoma nearcticum and Pseudodiplorchis americanus (see Tinsley, 1990). Polystoma nearcticum infects the urinary bladders of 2 closely related treefrogs, Hyla chrysoscelis and H. versicolor, which reside in forests and grassland habitats throughout the eastern United States (Tinsley, 1990; Bolek and Coggins, 1998; Du Preez et al., 2007; Muzzall and Kuczynski, 2017). Interestingly, the life cycle of Po. nearcticum is synchronized with the reproductive biology of its treefrog definitive hosts (Figure 7). During the spring, when treefrogs enter permanent ponds to breed, adult forms of Po. nearcticum that live in the frog’s urinary bladder begin laying unembryonated eggs concurrently with the oviposition activities of their treefrog definitive hosts. The eggs of Po. nearcticum are released into the pond in the frog’s urine, and over a period of 10 days the eggs develop and hatch into short-lived motile larvae. Once hatched, the larvae of Po. nearcticum must find and infect their tadpole hosts within 20 hours of hatching. Interestingly, because tadpoles do not possess a urinary bladder, larvae of the worms enter the gill chamber of their tadpole hosts, where they mature in weeks and begin releasing eggs into the pond. The second generation of eggs produced by the branchial (gill) generation of Po. nearcticum develop and hatch coinciding with the metamorphoses of their tadpole hosts. When tadpoles transform into froglets they develop a urinary bladder and the larvae from the second generation of eggs of Po. nearcticum enter the froglet’s cloaca and migrate into the urinary bladder (Figure 7). Once inside the urinary bladder of their treefrog definitive hosts, Po. nearcticum reaches sexual maturity and begins producing eggs when its treefrog hosts return to their breeding ponds the following spring.
In contrast to Polystoma nearcticum, Pseudodiplorchis americanus infects the urinary bladder of Couch’s spadefoot toads, Scaphiopus couchii, an amphibian species that lives in deserts and arid habitats throughout the southwestern United States (Tinsley, 1990). Unlike the treefrog hosts of Po. nearcticum, Couch’s spadefoot toads only enter temporary desert pools to mate and deposit eggs for approximately 21 hours per year (Tinsley, 1990). Since spadefoot toad tadpoles must complete metamorphosis in rapidly drying desert pools, they have one of the shortest developmental periods of any anuran species ranging from 7 to 20 days (Dodd, 2013). However, even with rapid metamorphosis, spadefoot toad tadpole mortality is often quite high in these desert pools, making tadpoles unreliable hosts for Ps. americanus. As a result, the transmission of Ps. americanus is confined to 1 to 3 nights each summer when the desert-adapted toads spawn. To overcome this temporal problem, selection has favored a dramatic modification in the life cycle of Pseudodiplorchis americanus. Instead of producing eggs that must develop for weeks in the external environment and infect tadpoles, the larvae of Ps. americanus complete their development inside the uterus of worms in the urinary bladder of spadefoot toads. Once spadefoot toads enter desert pools to spawn, the larvae hatch within seconds of being released with the toad’s urine into freshwater (Figure 7). When the larvae encounter a spadefoot toad in the water, they crawl up the chest of the amphibian and invade the nostrils. The larvae then migrate via the buccal cavity into the lungs where development occurs. Within a few weeks, the juvenile worms then migrate from the lungs by the intestine and cloaca into the urinary bladder. In the bladder, juvenile worms mature and then mate, accumulating new larvae in their uteri that will infect spadefoot toads the following year. Remarkably, the larvae of Ps. americanus appear to have specific adaptations for infecting adult spadefoot toads. For example, they are 2 to 4 times the size of larvae of any other species of polystomatid flatworms. Additionally, these giant larvae can swim for twice as long as larvae of Polystoma nearcticum, allowing them 2 days to encounter a spadefoot toad in water. Finally, the larvae of Ps. americanus can survive drying for up to an hour, which is likely an adaptation that allows the larvae of Ps. americanus to leave the water and crawl up the chest of their spadefoot hosts and enter the nasal passages (Tinsley and Earle, 1983).
The second example demonstrates how a generalist parasite, the tadpole pinworm, Gyrinicola batrachiensis, has a modified life cycle that appears to increase its reproductive success in different species of hosts. Gyrinicola batrachiensis infects the large intestine of tadpoles and has been reported from 18 species of frogs and toads (Pierce et al., 2018). Adult anurans are resistant to infections and (as noted above) tadpoles lose their pinworm infections when they metamorphose into adults, which in turn gives G. batrachiensis limited time for reproduction in its tadpole hosts. To make matters more complex, not all tadpole hosts are equal in terms of pinworm development and reproduction. For example, tadpoles of some anuran species metamorphose in just a few weeks (short developmental period) giving limited time for pinworm reproduction, while tadpoles of other anuran species take months to years (long developmental period) to metamorphose, giving pinworms more time for reproduction. However, pinworms cannot choose what species of tadpoles they will infect because all tadpoles become infected with G. batrachiensis when they accidentally ingest a pinworm egg on the pond bottom.
Investigation has shown that Gyrinicola batrachiensis exhibits 2 different but related lifestyles that appear to solve the problem for both short- and long-lived larval anurans. To overcome these constraints, G. batrachiensis has evolved 2 different reproductive strategies. The first strategy involves asexual reproduction, by parthenogenesis, when unmated female pinworms produce thick-shelled environmentally resistant eggs that are passed in tadpole feces to infect other tadpoles in the pond. The second strategy involves sexual reproduction by female and male pinworms, which results in female G. batrachiensis that produce 2 types of eggs: thick-shelled and thin-shelled. The thick-shelled eggs are released into the external environment to infect other tadpoles, which are similar to eggs produced by parthenogenic females. In contrast, thin-shelled eggs never leave the tadpole’s intestine and they are autoinfective, hatching quickly in the tadpole’s gut thus rapidly increasing the number of pinworms in a single tadpole.
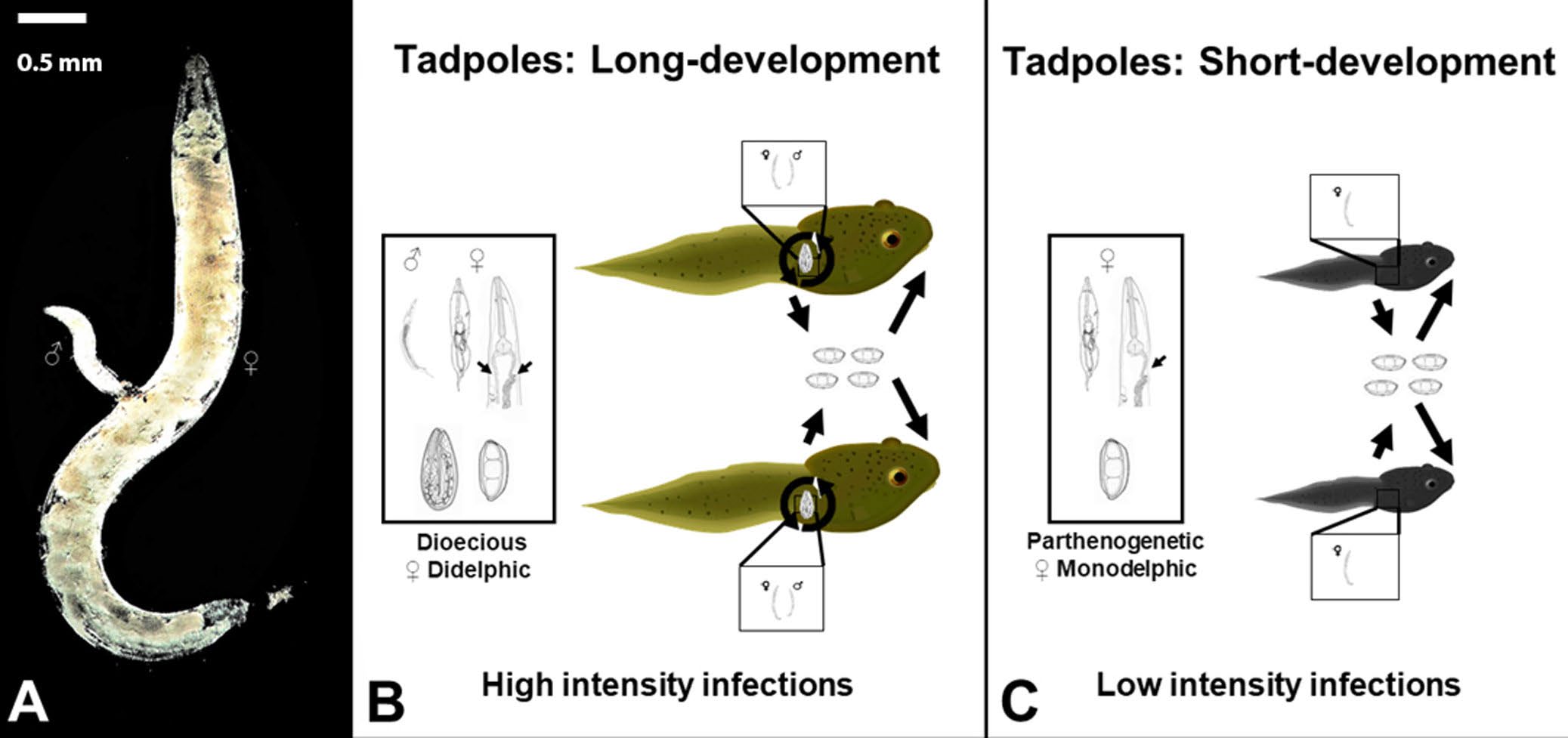
Figure 8. Example of plasticity in a direct life cycle of a generalist parasite Gyrinicola batrachiensis. A) A male (♂) in the process of mating with a female G. batrachiensis (♀). Scale bar = 0.5 mm. B) Dioecious reproductive strategy of G. batrachiensis in tadpoles with long developmental periods. Female worms are didelphic and produce thick-shelled and thin-shelled autoinfective eggs. As a result, tadpoles with long developmental periods have high intensities of G. batrachiensis. C) Parthenogenetic reproductive strategy of G. batrachiensis in tadpoles with short developmental periods. Female worms are monodelphic and only produce thick-shelled eggs. As a result, tadpoles with short developmental periods usually have much lower intensities of G. batrachiensis.
(Source: M. Bolek. License: CC BY-NC-SA 4.0.)
Production of thin-shelled autoinfective eggs varies according to the amphibian species and its tadpole developmental time (Figure 8). In tadpoles with short developmental periods that provide limited opportunities for pinworm recruitment and reproduction, pinworms can reproduce parthenogenetically (Adamson, 1981). Parthenogenetic pinworms are monodelphic and produce thick-shelled environmentally-resistant eggs. While parthenogenetic pinworms do not benefit from sexual recombination, reproduction via parthenogenesis increases the probability that the nematode offspring will infect another tadpole before their current host metamorphoses. Alternatively, in tadpoles with long developmental periods that allow Gyrinicola batrachiensis more time for development and reproduction, nematodes reproduce sexually (Adamson, 1981; Rhoden and Bolek, 2011; Childress et al., 2017; Pierce et al., 2018).
Female nematodes in tadpoles with long developmental periods are didelphic, producing thick-shelled environmentally resistant eggs in 1 uterine branch and thin-shelled autoinfective eggs in the second branch of the uterus. As a result of the autoinfective reproductive strategy, pinworms in long-developing tadpoles increase their numbers quickly and in the long run, a female worm can produce numerous reproductively active progeny inside a single tadpole host. So, although Gyrinicola batrachiensis might not always end up in their ideal host, that is, a long developing tadpole, they always try to make the most of their lot in life!
Literature Cited
Adamson, M. L. 1981. Development and transmission of Gyrinicola batrachiensis (Walton, 1929) (Pharyngodonidae: Oxyuroidea). Canadian Journal of Zoology 59: 1,351–1,367.
Andrews, J. A., J. N. Childress, T. J. Iakovidis, and G. J. Langford. 2015. Elucidating the life cycle and life history of Dero hylae (Naididae), a rare parasitic oligochaete from Florida tree frogs. Journal of Parasitology 10: 275–281. doi: 10.1645/14-608.1
Arias-Robledo, G., T. Stark, R. L. Wall, and J. R. Stevens. 2019. The toad fly Lucilia bufonivora: Its evolutionary status and molecular identification. Medical and Veterinary Entomology 33: 131–139. doi: 10.1111/mve.12328
Baker, M. R. 1979. The free-living and parasitic development of Rhabdias spp. (Nematoda: Rhabdiasidae) in amphibians. Canadian Journal of Zoology 57: 161–178. doi: 10.1139/z79-014
Bolek, M. G. 1997. Seasonal occurrence of Cosmocercoides dukae and prey analysis in the blue-spotted salamander, Ambystoma laterale, in southeastern Wisconsin. Journal of the Helminthological Society of Washington 64: 292–295.
Bolek, M. G., and J. R. Coggins. 1998. Endoparasites of Cope’s gray treefrog, Hyla chrysoscelis, and western chorus frog, Pseudacris t. triseriata, from southeastern Wisconsin. Journal of the Helminthological Society of Washington 65: 212–218.
Bolek, M. G., and J. R. Coggins. 2002. Observations on myiasis by the calliphorid, Bufolucilia silvarum, in the Eastern American toad (Bufo americanus americanus) from southeastern Wisconsin. Journal of Wildlife Diseases 38: 598–603. doi: 10.7589/0090-3558-38.3.598
Bolek, M. G., and J. J. Janovy, Jr. 2008. Alternative life cycle strategies of Megalodiscus temperatus in tadpoles and metamorphosed anurans. Parasite 15: 396–401. doi: 10.1051/parasite/2008153396
Bolek, M. G., and J. J. Janovy, Jr. 2007a. Evolutionary avenues for and constraints on the transmission of frog lung flukes (Haematoloechus spp.) in dragonfly second intermediate hosts. Journal of Parasitology 93: 593–607. doi: 10.1645/GE-1011R.1
Bolek, M. G., and J. J. Janovy, Jr. 2004. Observations on myiasis by the calliphorids, Bufolucilia silvarum and Bufolucilia elongata, in wood frogs, Rana sylvatica, from southeastern Wisconsin. Journal of Parasitology 90: 1,169–1,171. doi: 10.1645/GE-246R
Bolek, M. G., and J. J. Janovy, Jr. 2007b. Small frogs get their worms first: The role of non-odonate arthropods in the recruitment of Haematoloechus coloradensis and Haematoloechus complexus in newly metamorphosed northern leopard frogs, Rana pipiens, and Woodhouse’s toads, Bufo woodhousii. Journal of Parasitology 93: 300–312. doi: 10.1645/GE-1010R.1
Bolek, M. G., S. D. Snyder, and J. J. Janovy, Jr. 2009. Alternative life cycle strategies and colonization of young anurans by Gorgoderina attenuata in Nebraska. Journal of Parasitology 95: 604–615. doi: 10.1645/GE-1813.1
Bolek, M. G., H. A. Stigge, and K. D. Gustafson. 2016. The iron wheel of parasite life cycles: Then and now! In J. J. Janovy, Jr., and G. W. Esch, eds. A Century of Parasitology: Discoveries, Ideas and Lessons Learned by Scientists Who Published in the Journal of Parasitology, 1914–2014. Wiley, London, United Kingdom, p. 131–147.
Bolek, M. G., H. R. Tracy, and J. J. Janovy, Jr. 2010. The role of damselflies (Odonata: Zygoptera) as paratenic hosts in the transmission of Halipegus eccentricus (Digenea: Hemiuridae) to anurans. Journal of Parasitology 96: 724–735. doi: 10.1645/GE-2365.1
Childress, J. N., C. S. Rogers, M. G. Bolek, and G. J. Langford. 2017. Reproductive plasticity in the nematode Gyrinicola batrachiensis: Does an intermediate reproductive strategy exist in sexually reproducing, didelphic pinworms? Journal of Parasitology 103: 663–668. doi: 10.1645/17-30
Clopton, R. E., J. J. Janovy, Jr., and T. J. Percival. 1992. Host stadium specificity in the gregarine assemblage parasitizing Tenebrio molitor. Journal of Parasitology 78: 334–337.
Dodd, Jr., K. C. 2013. Frogs of the United States and Canada. Johns Hopkins University Press, Baltimore, Maryland, United States, 982 p.
Du Preez, L. H., O. Verneau, and T. S. Gross. 2007. Polystoma floridana n. sp. (Monogenea: Polystomatidae) a parasite in the green tree frog, Hyla cinerea (Schneider), of North America. Zootaxa 1663: 33–45. doi: 10.11646/zootaxa.1663.1.3
Gibson, D. I., and R. A. Bray. 1994. The evolutionary expansion and host-parasite relationship of Digenea. International Journal for Parasitology 24: 1,213–1,226. doi: 10.1016/0020-7519(94)90192-9
Hartigan A., I. Fiala, I. Dyková, K. Rose, et al. 2012. New species of Myxosporea from frogs and resurrection of the genus Cystodiscus Lutz, 1889 for species with myxospores in gallbladders of amphibians. Parasitology 139: 478–496. doi: 10.1017/S0031182011002149
Janovy, Jr., J. J., J. Detwiler, S. Schwank, M. G. Bolek, et al. 2007. New and emended descriptions of gregarines from flour beetles (Tribolium spp. and Palorus subdepressus: Coleoptera, Tenebrionidae). Journal of Parasitology 93: 1,155–1,170. doi: 10.1645/GE-1090R.1
Jirků, M., M. G. Bolek, C. M. Whipps, J. J. Janovy, Jr., et al. 2006. A new species of Myxidium (Myxosporea: Myxidiidae), from the western chorus frog, Pseudacris triseriata triseriata, and Blanchard’s cricket frog, Acris crepitans blanchardi (Hylidae) from eastern Nebraska USA: Morphology, phylogeny and critical comments on amphibian Myxidium taxonomy. Journal of Parasitology 92: 611–619. doi: 10.1645/GE-728R.1
Jirků, M., I. Fiala, and D. Modrý. 2007. Tracing the genus Sphaerospora: Rediscovery, redescription and phylogeny of the Sphaerospora ranae (Morelle, 1929) n. comb. (Myxosporea, Sphaerosporidae), with emendation of the genus Sphaerospora. Parasitology 134: 1,727–1,739. doi: 10.1017/S0031182007003241
Krull, H. W. 1952. Studies on the biology of Dicrocoelium dendriticum (Rudolphi, 1819) Looss, 1899 (Trematoda: Dicrocoeliidae), including its relation to the intermediate host, Cionella lubrica (Müller), VII: The second intermediate host of Dicrocoelium dendriticum. Cornell Veterinarian 42: 603–604.
Lafferty, K. D. 1999. The evolution of trophic transmission. Parasitology Today 15: 111–115.
Lafferty, K. D., and A. M. Kuris. 2002. Trophic strategies, animal diversity and body size. Trends in Ecology and Evolution 17: 507–513. doi: 10.1016/S0169-5347(02)02615-0
Langford, G. J., and J. J. Janovy, Jr. 2009. Comparative life cycles and life histories of North American Rhabdias spp. (Nematoda: Rhabdiasidae): Lungworms from snakes and anurans. Journal of Parasitology 95: 1,145–1,155. doi: 10.1645/GE-2044.1
Langford, G. J., and J. J. Janovy, Jr. 2013. Host specificity of North American Rhabdias spp. (Nematoda: Rhabdiasidae): Combining field data and experimental infections with a molecular phylogeny. Journal of Parasitology 99: 277–286. doi: 10.1645/GE-3217.1
Muzzall, P. M., and M. C. Kuczynski. 2017. Helminths of the eastern gray treefrog, Hyla versicolor (Hylidae), from a pond in southwestern lower Michigan, USA. Comparative Parasitology 84: 55–59. doi: 10.1654/1525-2647-84.1.55
Nickol, B. B. 2003. Is postcyclic transmission underestimated as an epizootiological factor for acanthocephalans? Helminthologica 40: 93–95.
Nickol, B. B., and R. W. Heard. 1973. Host parasite relationships of Fessisentis necturorum (Acanthocephala: Fessisentidae). Proceedings of the Helminthological Society of Washington 40: 204–208.
Parker, G. A., J. C. Chubb, M. A. Ball, and G. N. Roberts. 2003. Evolution of complex life cycles in helminth parasites. Nature 425: 480–484. doi: 10.1038/nature02012
Pierce, C. C., R. P. Shannon, and M. G. Bolek. 2018. Distribution and reproductive plasticity of Gyrinicola batrachiensis (Oxyuroidea: Pharyngodonidae) in tadpoles of five anuran species. Parasitology Research 117: 461–470. doi: 10.1007/s00436-017-5723-4
Poulin, R. 2007. Evolutionary Ecology of Parasites, 2nd edition. Princeton University Press, Princeton, New Jersey, United States, 360 p.
Poulin, R., and T. H. Cribb. 2002. Trematode life cycles: Short is sweet? Trends in Parasitology 18: 176–183. doi: 10.1016/s1471-4922(02)02262-6
Rhoden, H. R., and M. G. Bolek. 2011. Distribution and reproductive strategies of Gyrinicola batrachiensis (Oxyuroidea: Pharyngodonidae) in larvae of eight species of amphibians from Nebraska. Journal of Parasitology 97: 629–635. doi: 10.1645/GE-2670.1
Rhoden, H. R., and M. G. Bolek. 2012. Helminth and leech community structure in tadpoles and caudatan larvae of two amphibian species from western Nebraska. Journal of Parasitology 98: 236–244. doi: 10.1645/GE-2771.1
Rhoden, H. R., and M. G. Bolek. 2015. Helminth community structure in tadpoles of northern leopard frogs (Rana pipiens) and Woodhouse’s toads (Bufo woodhousii) from Nebraska. Parasitology Research 114: 4,685–4,692. doi: 10.1007/s00436-015-4716-4
Runey, W. M., G. L. Runey, and F. H. Lauter. 1978. Gametogenesis and fertilization in Rhabdias ranae Walton 1929, I: The parasitic hermaphrodite. Journal of Parasitology 64: 1,008–1,014.
Schell, S. C. 1962. The life history of Telorchis bonnerensis Waitz (Trematoda: Reniferidae), a parasite of the long-toed salamander, Ambystoma macrodactylum Baird. Transactions of the American Microscopical Society 81: 137–146.
Smith-Trail, D. R. 1980. Behavioral interactions between parasites and hosts: Host suicide and evolution of complex life cycles. American Naturalist 116: 77–91. doi: 10.1086/283612
Stigge, H. A., and M. G. Bolek. 2016. Anuran host species influence site fidelity of Halipegus occidualis. Journal of Parasitology 102: 47–53. doi: 10.1645/15-790
Stigge, H. A., and M. G. Bolek. 2015. The alteration of life history traits and increased success of Halipegus eccentricus through the use of a paratenic host: A comparative study. Journal of Parasitology 101: 658–665. doi: 10.1645/15-793
Tantawi, T. I., and T. Whitworth. 2014. First record of Lucilia bufonivora Moniez, 1876 (Diptera: Calliphoridae) from North America and key to North American species of the L. bufonivora species group. Zootaxa 3881: 101–124. doi: 10.11646/zootaxa.3881.2.1
Taylor, C. N., K. L. Oseen and R. J. Wassersug. 2004. On the behavioral response of Rana and Bufo tadpoles to echinostomatoid cercariae: Implications to synergistic factors influencing trematode infections in anurans. Canadian Journal of Zoology 82: 701–706. doi: 10.1139/z06-158
Thiemann, G. W., and R. J. Wassersug. 2000. Biased distribution of trematode metacercariae in the nephric system of Rana tadpoles. Journal of Zoology, London 252: 534–538.
Tinsley, R. C. 1990. Opportunism in parasite life cycles. In C. J. Barnard and J. M. Behnke, eds. Parasitism and Host Behaviour. Burgess Science Press, London, United Kingdom, p. 158–192.
Tinsley, R. C., and C. M. Earle. 1983. Invasion of vertebrate lungs by the polystomatid monogeneans Pseudodiplorchis americanus and Neodiplorchis scaphiopodis. Parasitology 83: 501–518. doi: 10.1017/S0031182000050691
Zamparo, D., and D. R. Brooks. 2003. Phylogenetic systematic assessment of the Aspidobothrea (Platyhelminthes, Neodermata, Trematoda). Zoologica Scripta 32: 83–93.
Zumpt, F. 1965. Myiasis in Man and Animals in the Old World: A Textbook for Physicians, Veterinarians, and Zoologists. Butterworths, London, United Kingdom, 267 p.
Adjective
From Greek: koilos = hollow; zoon = animal
Definition: Living in the lumen of a hollow organ, that is, the intestine
Adjective
From Greek: histos = tissue; zoon = animal
Definition: Dwelling within the tissues of a host
Adjective
Definition: Refers to a parasite that is limited to a single host species
Adjective
From Greek: stenos = narow; xenos = host
Definition: Refers to a parasite that has a narrow host range
Adjective
Definition: Refers to a parasite that infects unrelated hosts
Definition: Refers to hosts only being susceptible to infection by a particular parasite at a specific developmental stage
Adjective
From Greek: monos = one; xenos = guest/host/stranger
Definition: Living within a single host during a parasite's life cycle
Adjective
From Greek: heteros = different; xenos = host/guest/stranger
Definition: Having more than one host during a parasite's life cycle
Adjective
From Greek: heteros = different; genesis = descent
Definition: Pertaining to meiotic chromosome pairing in hybrids when pairs are derived from different ancestors
Adjective
From Greek: monos = one; genesis = beginning
Definition 1: Pertaining to monogenesis
Definition 2: Designates parasites with a simple direct life cycle that is completed in one host
Definition 3: Producing offspring of one sex by arrhenogenesis or thelygenesis
Noun
From Greek: myia = fly; iasis = morbid condition
Definition: A condition deriving from invasion by dipterous larvae
Definition: A parasite that cannot exist without a host during all or some portion of the life cycle. see facultative parasite
Noun
Adjective: heterogonous
From Greek: heteros = different; gonos = seed
Definition 1: Study of relative growth
Definition 2: Alternation of generations
Definition 3: Both males and females present in a colony
Noun
From Greek: endon = within; tokos = birth
Definition: A form of reproduction in which the eggs develop within the body of the mother
Noun
Adjective: polyembryonic
From Greek: polys = many; embryon = fetus
Definition: The formation of multiple embryos from a single egg
Noun
Adjective: parthenogenetic
From Greek: parthenos = virgin; genesis = origin
Definition: The development of an individual from an unfertilized egg
Noun
From Greek: autos = self
From Latin: inficere = to taint
Definition: Infection of a host by microorganisms or parasites produced within or upon the body of the same individual host
Adjective
From Greek: dis = twice; delphys = womb
Definition: Having two uteri

