6 Behavioral Parasitology
Megan Wise de Valdez
Introduction
As pointed out in the previous sections, parasites have an intimate relationship with their hosts and can affect many aspects of their host’s biology. By definition, parasites live at the expense of their host, causing some type of physical or physiological damage, but they can also affect host behaviors. Throughout this section, the who, what, when, where, why, and how of parasite manipulation of host behaviors will be investigated.
To categorize before moving on to examples, direct versus indirect effects on host behaviors should be described. Parasites can influence host behaviors directly through the physical presence of the parasite within a host or they may indirectly influence host behavior when potential hosts exhibit behaviors in order to avoid becoming infected with the parasite. Some examples of these parasite-avoidance behaviors are swatting, moving to a different habitat, feeding/foraging at specific times of day, and grooming (see Moore, 2002 for full review). Although these indirect effects on host behavior are interesting and certainly worthy of study, the direct effects of parasites on host behaviors are most salient. Parasite-induced behavioral alteration/modification refers to a behavioral change in a host that is caused by the presence of a parasite; there is no underlying assumption that the behavioral change is advantageous to either the host or the parasite. Note that the words modification and alteration can be used interchangeably. Parasite manipulation of behavior implies that the parasite is actively changing a host behavior in order to benefit itself. In the rest of this chapter, the basic principles of parasite-induced behavioral modifications will be established by exhibiting case-studies from the scientific literature to help answer 3 basic questions:
1) Why are there parasite-induced behavioral modifications of hosts?
2) Which host behaviors or traits are likely to be altered?
3) When are host behaviors altered?
At the end of the chapter, there is a set of more advanced questions for those students who may want to delve deeper into the complexity of this aspect of host-parasite relationships.
Learning Objectives
- Apply the scientific method to address questions about parasite-induced modification of host behaviors.
- Analyze examples in the scientific literature to learn how scientists have experimentally addressed questions about parasite manipulation of host behaviors.
- Be able to provide some classic examples of parasite-induced modification of host behaviors.
- Understand the evolutionary principles of parasite manipulation of host behaviors.
- Understand the types of host behaviors likely to be altered in relation to the parasites’ life cycles.
- Think critically about host-parasite relationships yet to be investigated from a behavioral standpoint.
Why Are There Parasite-Induced Behavioral Modifications of Hosts?
There is no simple answer to this question, but there are 3 primary hypotheses: 1) The altered behavior is a side-effect of infection, 2) the host benefits from the altered behavior (host-adaptation), and 3) the parasite benefits from the altered behavior (parasite-adaptation) (Poulin, 2010; Moore, 2013). Each will be discussed in turn.
Behavioral Changes as Side-Effects of Infection
Behavioral alterations as side-effects of infection appears to be the simplest answer because an infected host is expected to act sick, especially if the behavioral changes appear to be of no obvious advantage to either host or parasite. However, unless the hypothesis is tested it should not be used as the default explanation. Wise de Valdez (2007) conducted a study to determine whether parasite-induced behavioral changes were a side effect of infection or if they were advantageous to the parasite. The host-parasite system used was mermithid-mosquito system. Mermithids are nematode worms that can use aquatic mosquito larvae for development where they then emerge to a free-living state. During their development they grow and eventually take over much of the space inside the mosquito larvae after which they exit the mosquito larvae, killing it. Wise de Valdez (2006) found that mermithid nematodes made their mosquito larvae hosts less active and it is tempting to hypothesize that the change in activity levels is simply a side effect of the worm filling up the entire space of the mosquito larvae. An alternative hypothesis would be that the behavioral changes benefit the parasite by making the mosquito less likely to be eaten by a predator and thus survive long enough to emerge to its free-living stage. To test these hypotheses, Wise de Valdez (2007) experimentally infected Aedes aegypti mosquito larvae with mermithid nematodes and confirmed that their activity levels were lower than those without an infection. Predation experiments were then conducted using the predatory mosquito larva Toxorhynchites rutilus and it was found that the predator consumed both infected and uninfected larvae at equal rates. Therefore, the experiment supported the hypothesis that the behaviors are a side effect of infection, and the reduction in activity levels did not appear to benefit the parasite because they were eaten just as often as uninfected mosquitoes.
However, a singular set of experiments supporting a hypothesis does not necessarily make the hypothesis definitive. The important thing is that data were gathered and allowed the investigators to begin to make more educated assumptions about a system. Future scientists could use this study to develop new hypotheses that might lead to other conclusions after testing. This is what is so great about science, new hypotheses can always be tested! When the host’s behaviors don’t necessarily fit the classic sick behavior or when entirely new and unexpected behaviors are observed, other explanations may be sought.
Notes on the Scientific Method
No matter in what stage someone is in their scientific career, all employ the scientific method, or at least parts of it, when embarking on new areas of study. In fact, most scientists have internalized the process as they use it on a daily basis. It is helpful to periodically revisit the formal nature of the scientific method. Thus, because for many students, this will be the first time considering parasite manipulation of host behaviors, you may approach it as new scientists using the scientific method. It all starts with an observation followed by a question (or many). Then use your previous scientific knowledge, or read a bit more, to come up with an educated answer to that question: The hypothesis. All good hypotheses must be testable. Now, the hypothesis may or may not be the right answer to the question; it is only a best guess based on previous understanding of a system. Therefore, to determine if the hypothesis is correct, the hypothesis must be tested, and data must be gathered through observational or experimental studies. Through data analysis, it can then be confirmed whether the hypothesis may be supported or rejected.
Host Adaptation: The Host Benefits from Altered Behaviors
Another hypothesis to consider how to answer the why of behavioral alterations is that the host could benefit in some way from a change in behavior. The altered behavior would then be considered a host adaptation. An adaptation is a character that increases the fitness of an organism and fitness is the ability of an organism to survive long enough to successfully reproduce. Adaptations arise through natural selection; individuals that exhibit a particular trait survive and reproduce more than individuals that do not exhibit that trait. The parasite-induced behavioral changes of an infected host would be a host adaptation if they help to reduce or rid the host of parasites and thereby increase host survival and reproductive capacity (that is, its fitness).
Unusual foraging habits that are a form of self-medication have been observed as a behavioral change that benefits the host. For instance, chimpanzees will eat medicinal plants that are not part of its normal diet (Moore, 2013, citing Huffman, 1997). Caterpillars infected with a parasitoid fly will switch plant food source and increase its survival (Moore, 2013, citing Karban and English-Loeb, 1997). Two other classic behavioral strategies that have evolved in some infected hosts in response to parasitism are known as behavioral fever and behavioral chills which are characterized by movement of infected hosts to a higher or lower than normal temperature to rid themselves or reduce the impact of a pathogen (see Moore, 2002 for review). Both of these are most likely to occur in organisms that cannot regulate their temperature metabolically. Metabolic fever in endotherms is well documented. It induces a behavioral change that brings the afflicted individuals to a habitat with a particular temperature. Müller and Schmid-Hempel (1993) found that bumblebees infected with parasitoid fly larvae remained outside the hive where it was colder and when given a choice they spent more time in cold areas than uninfected bumble bees (behavioral chills). By altering their behavior to choose colder temperatures, these infected bumblebees lived longer and had fewer fully-developed parasitoids that the infected bumblebees that were kept at normal temperatures. A study by Watson and colleagues (1993) showed that house flies infected with a fungal pathogen that spent at least 8 hours in 40 °C temperature shortly after infection survived longer than those that did not. Interestingly, this behavior did not benefit the house fly if the infection was more advanced (after 5 days post infection). Even more interesting, and evidence that parasite-induced behavioral alterations are complex, was that the flies that did not successfully employ behavioral fever moved to cooler temperatures, a behavior that benefited the fungal parasite; cooler temperatures enhanced the propagation of the parasitic fungus!
Parasite Adaptation: The Parasite Benefits from Altered Behaviors
In the first half of the 20th century, several researchers proposed that parasites may be able to alter the behaviors of their hosts in ways that increase their transmission success (Lefèvre et al., 2009 citing Cram, 1931; Van Dobben, 1952). Later, in the 1970s and 1980s, researchers provided some of the first empirical evidence that intermediate hosts infected with parasites exhibit different behaviors than those that were uninfected. Furthermore, the infected hosts were more likely to be consumed by the next host in their life cycle, thereby increasing transmission success (Hindsbo, 1972; Bethel and Holmes, 1973; 1974; 1977; Moore, 1983). These studies involved acanthocephalan parasites and their crustacean intermediate hosts. Bethel and Holmes (1973; 1977) demonstrated that small aquatic crustaceans, Hyalella azteca and Gammarus lacustrus, infected with 1 of 2 different species of acanthocephalans, Polymorphus paradoxus or Corynosoma constrictum, exhibit behaviors that move them to areas where their habitat overlaps with the feeding area of the parasites’ definitive host and may make them more conspicuous. Through predation experiments using birds and muskrats, they found that infected crustaceans were more vulnerable to predation by mallard ducks and accidental ingestion by muskrats (both definitive hosts) than uninfected crustaceans. A study by Moore (1983) showed that the juvenile stage of Plagiorhynchus cylindraceus induces risky behavior of its isopod pill bug host, thereby causing it to be more conspicuous to its definitive host predator, the European starling (the details of this study will be discussed later in the chapter).
These initial studies kick-started research on parasite manipulation of host behaviors in earnest and since then researchers have found examples across all parasite taxa: protozoan parasites, plathyhelminth parasites in the classes Trematoda (flukes) and Cestoda (tapeworms), acanthocephalans, nematodes, nematomorphs, and parasitic arthropods (see reviews by Adamo, 1997; Moore, 2002; Lefèvre et al., 2009; Hughes et al., 2012). Discovering the adaptive nature of these behavioral alterations in a scientifically sound way became well established (Poulin, 1995; 2010). Furthermore, the types of questions being asked about parasite-induced behavioral alterations have expanded to include more complex questions (see the Advanced Questions below). For the remainder of the chapter the primary focus will be on the hypothesis of parasite manipulation of behaviors as parasite adaptations.
Which Host Behaviors or Traits are Likely to Be Altered, and When?
Life Cycles and Transmission Routes
In order to understand the adaptive nature of a parasite-induced behavioral change, the life cycle of the parasite in question must be understood. The parasite life cycle plays a major role in which host is likely to be manipulated and which behaviors are manipulated. Parasites with complex life cycles have multiple hosts; 1 or more intermediate hosts which are infected with an immature stage of the parasite, and a definitive host in which the parasite reaches sexual maturity. Parasites with simple life cycles have only 1 host, and parasitoid life cycles are unique in that 1 host is always killed by the parasite as it emerges to a free-living stage. Each life cycle has different requirements for how the parasite moves within the environment to reach a reproductive stage. Parasites with complex life cycles require movement from 1 host to the next. This movement can be up the food chain where 1 host lower on the food chain is consumed by the next host in the life cycle that is higher in the food chain (trophic transmission; Figure 1A). Movement can be through a vector, where 1 host (the vector) transmits the parasite to the next host (often via a bite) without being killed (vector-borne transmission; Figure 1B). Additionally, some parasites with complex or simple life cycles might require a host to bring them to a specific habitat where their eggs or larvae might be deposited (Figure 1C). Parasites with simple life cycles (1 host) are interesting because they may live their entire life within the single host or they may have 1 or more free-living stages, spending only part of their life cycle in the host. Some of these single-host parasites may use their host as a direct nutritional resource (Figure 1D), especially parasitoids, that usually consume much of their host in order to develop to their free-living stage. All of these different life cycles and transmission requirements open the door for some interesting ways in which parasite-induced behavioral modifications are manifested.
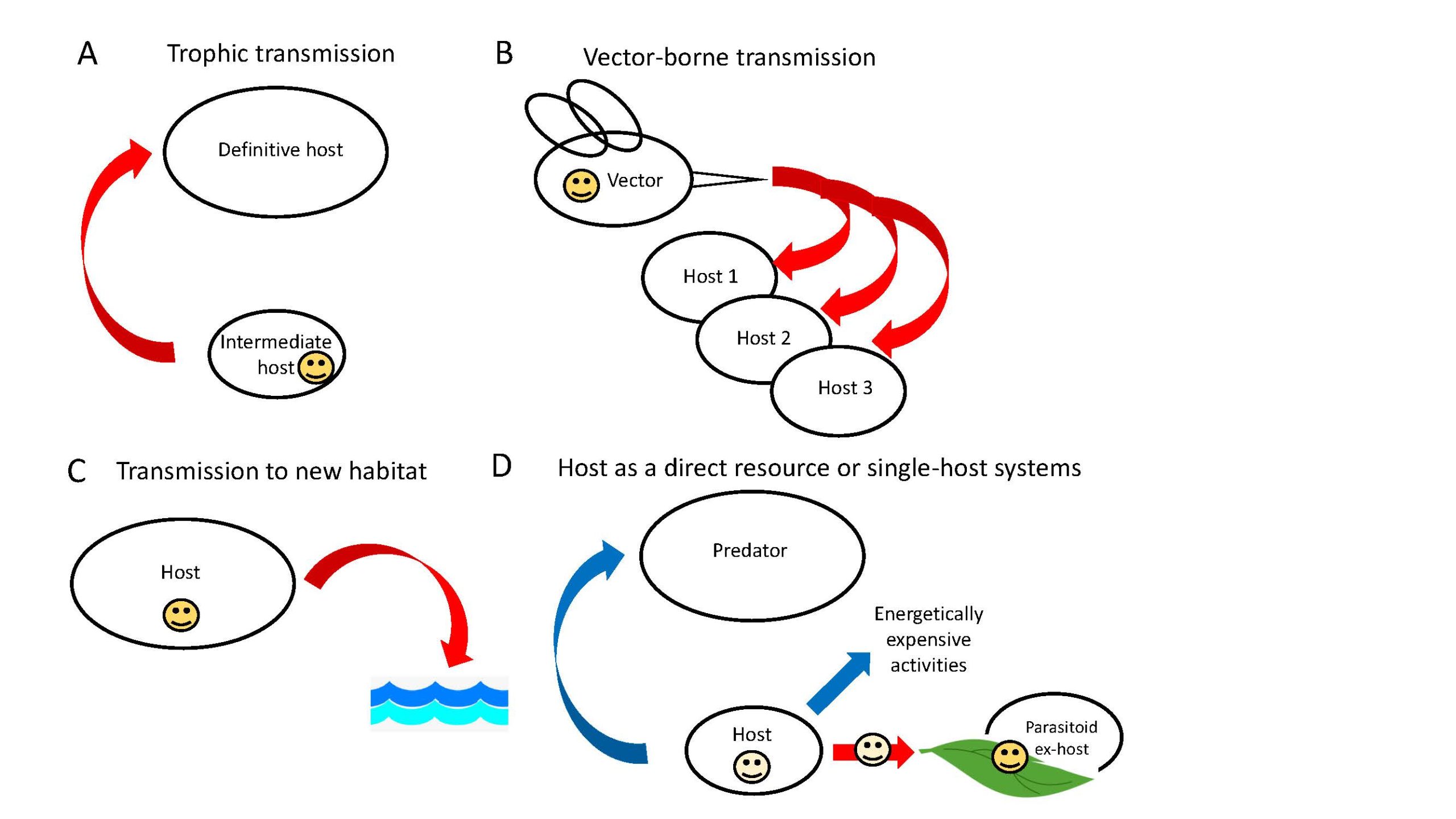
Figure 1. Presented are 4 main scenarios in which behavioral alterations have been seen. The smiley face is the parasite and the arrows indicate the stage in the life cycle where behavioral alterations are likely to occur. Red arrows indicate behaviors that increase the likelihood and blue arrows indicate behaviors that decrease the likelihood. A) Trophic transmission: Trophically-transmitted parasite behaviors of the intermediate host should be altered to increase transmission to the next host. B) Vector-borne transmission: In vector-borne parasite transmission, behaviors of the vector should be altered to increase transmission to multiple hosts or to increase the parasite load delivered. C) Transmission to a new habitat: Parasites that require delivery to a new environment, either themselves or their propagules, should manipulate the host to bring it to the appropriate habitat. D) Hosts as a direct resource: Parasites that use a host as a direct nutritional resource, usually parasitoids, should modify host behaviors to increase nutritional access or to prolong its survival and in some cases to elicit postemergence protection. Note: These scenarios are not mutually exclusive.
(Source: Adapted from Poulin, 2010. License: CC BY-NC-SA 4.0.)
Trophic Transmission
In complex life cycles where trophic transmission is required, it would be expected that the host likely to be manipulated would be the intermediate host and the altered behaviors should result in an increase in consumption of that intermediate host by the next host in the life cycle (Figure 1A). Even these expectations have their nuances; the behaviors manipulated will be different if the next host is a natural predator or if the next host is not a natural predator of the intermediate host. If the intermediate host is a natural food source of the next host in the life cycle, it would be expected that the parasite would alter its normal predator avoidance behaviors. For example, the intermediate hosts of Toxoplasma gondii are rats and the definitive hosts, cats, are a natural predator. Normally, rats find cat urine odor repulsive. This is a natural defense mechanism that elicits an avoidance behavior. However, when infected with T. gondii rats are attracted to cat urine and might even seek out the cat which should theoretically increase the rate of predation on infected rats and thereby promote trophic transmission (Berdoy et al., 2000). On the other hand, if the intermediate host is not a regular food source of the next host in the life cycle, parasites might manipulate behaviors that increase the contact these hosts have with their non-natural predator. For example, the trematode parasite Dicrocoelium dendriticum uses an ant as its intermediate host. In order for the life cycle to be completed, the ant harboring the juvenile trematode must be consumed by a grazing herbivore (usually a sheep or cow) which does not intentionally consume ants. The parasite manipulates the behavior of the ant in order to increase contact with the grazing definitive host. Ants infected with D. dendriticum act normally during the day but when the temperature drops, they climb to the top of blades of grass and clamp down with their mandibles. The ants are unable to release until the temperature rises again, thus positioning themselves to be eaten by grazing definitive hosts (Anokhin, 1966). Another extraordinary example that is quite evolutionarily complex is a nematode that not only causes the posterior end of an ant to turn red, but also manipulates the ant to hang out near a cluster of red berries. Yanoviak and fellow researchers (2008) conducted predation experiments and found that this manipulation of the phenotype and climbing near berries increased predation by the definitive host, frugivorous (fruit-eating) birds, that do not normally consume ants.
Included above are brief descriptions of just a few of the many studies that support the hypothesis that infected intermediate hosts behave differently than uninfected hosts and that these behavioral changes may be adaptive by increasing trophic transmission to the next host. However, many studies reported in the scientific literature (see review in Moore, 2002) have not provided experimental evidence that definitively supports that hypothesis. The reason these studies are less frequent in the literature is that they are simply hard to do. Pick any life cycle illustrated in this book and imagine what it would take to study the primary questions of parasite-induced behavioral changes. Not only would it first need to be established that the behaviors of infected and uninfected hosts differ, but then the next host in the life cycle would need to be included to determine if they became infected more often due to this behavior. Sometimes that next host in the life cycle is an animal that simply can’t be used in experiments (think humans, large carnivores) or may be uncooperative in experimental arenas. Despite this difficulty there are studies out there. Following is a detailed description of one of the seminal works that provides experimental evidence of parasite manipulation of hosts in a trophically transmitted parasite system.
Stop and Think
Before reading further, take a look at the life cycle (Figure 2) and think about what you already know about pillbugs and birds. Where do they live? How do they behave? What behaviors might be targeted by the parasite that might help it reach the starling? By doing this you are starting to formulate one or more hypotheses. How might these hypotheses be tested?
Moore (1983) investigated the acanthocephalan parasite Plagiorhynchus cylindraceus and the behavioral manipulation of its intermediate host Armadillidium vulgare (common pillbug). The life cycle of P. cylindraceus requires that the intermediate host, the pillbug, be eaten by the definitive host, the European starling (Sturnus vulgarus; Figure 2).
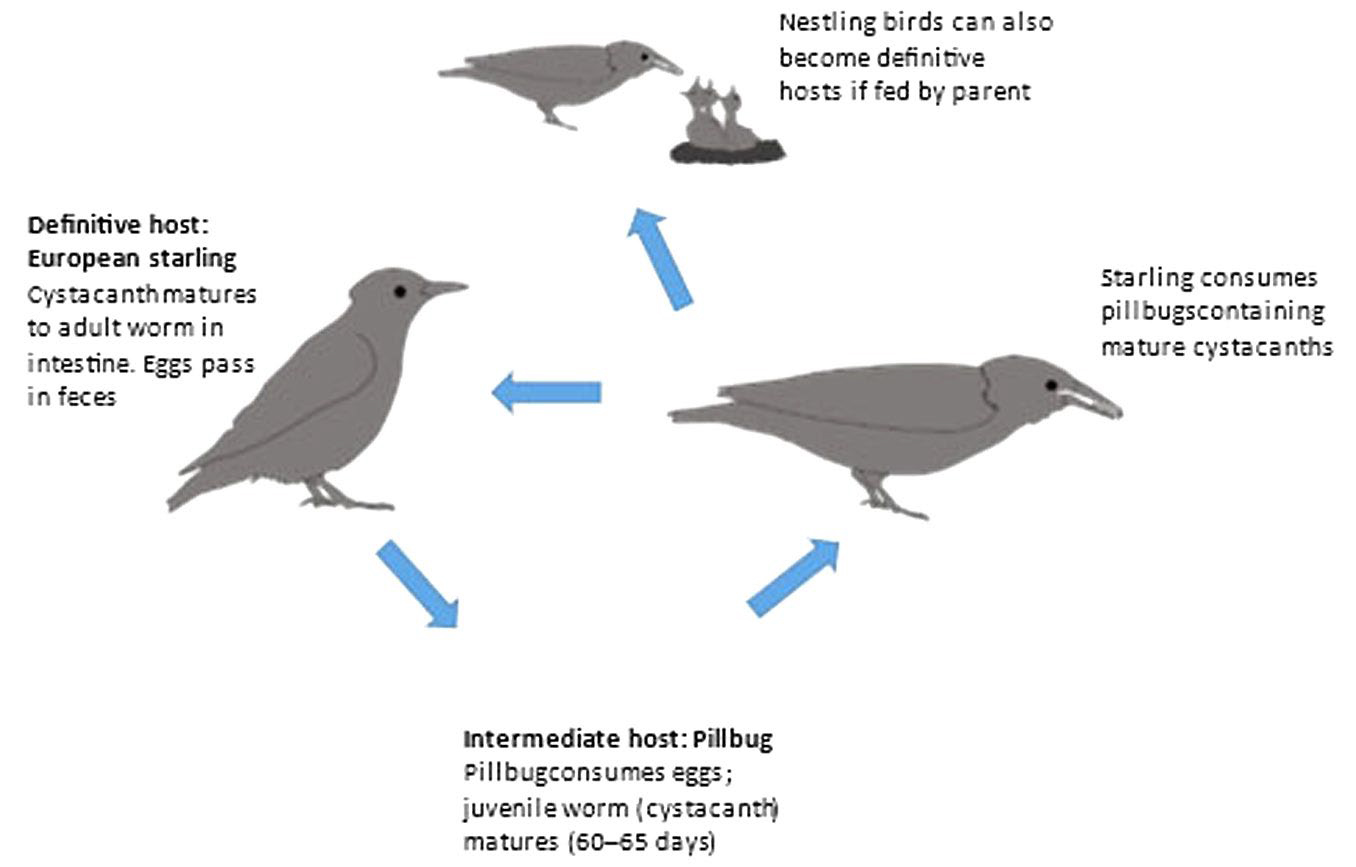
Figure 2. Life cycle of the acanthocephalan parasite Plagiorhynchus cylindraceus.
(Source: M. Wise de Valdez. License: CC BY-NC-SA 4.0.)
Moore conducted both laboratory and field experiments to investigate this host-parasite system. For this example, it is interesting to consider how 2 primary questions were answered: 1) Do infected pillbugs behave differently from uninfected pillbugs, and 2) Are infected pillbugs more likely to be eaten by starlings?
In order to answer the first question, “Do infected pillbugs behave differently from uninfected pillbugs?” Moore experimentally infected pillbugs and sham-infected others (Figure 3A). Sham infection is when the researcher treats the control animals similarly during the infection experiments but does not include the actual parasite. In behavioral experiments it is important to institute multiple controls in order to ensure that behavioral differences observed are the result of the parasitic infection and not a difference in treatment of the organisms. Another important thing to note is that the pillbugs in the intentionally-infected group may not always become infected. Exposure to parasite eggs does not ensure that the infection will take. For this reason, the 2 groups are referred to as exposed and unexposed (Figure 3A).
Because pillbugs are normally found in areas of high moisture and under leaf litter, bark, or rocks, and because these habitats also provide protection from potential visual predators, Moore chose to look at behaviors associated with habitat preference (humidity, shelter, substrate, and light) and overall activity level of the pillbugs. Moore set up several arenas to test habitat preference of infected and uninfected pillbugs (Figure 3B-E) and one to determine activity level (distance moved and time resting; Figure 3F).
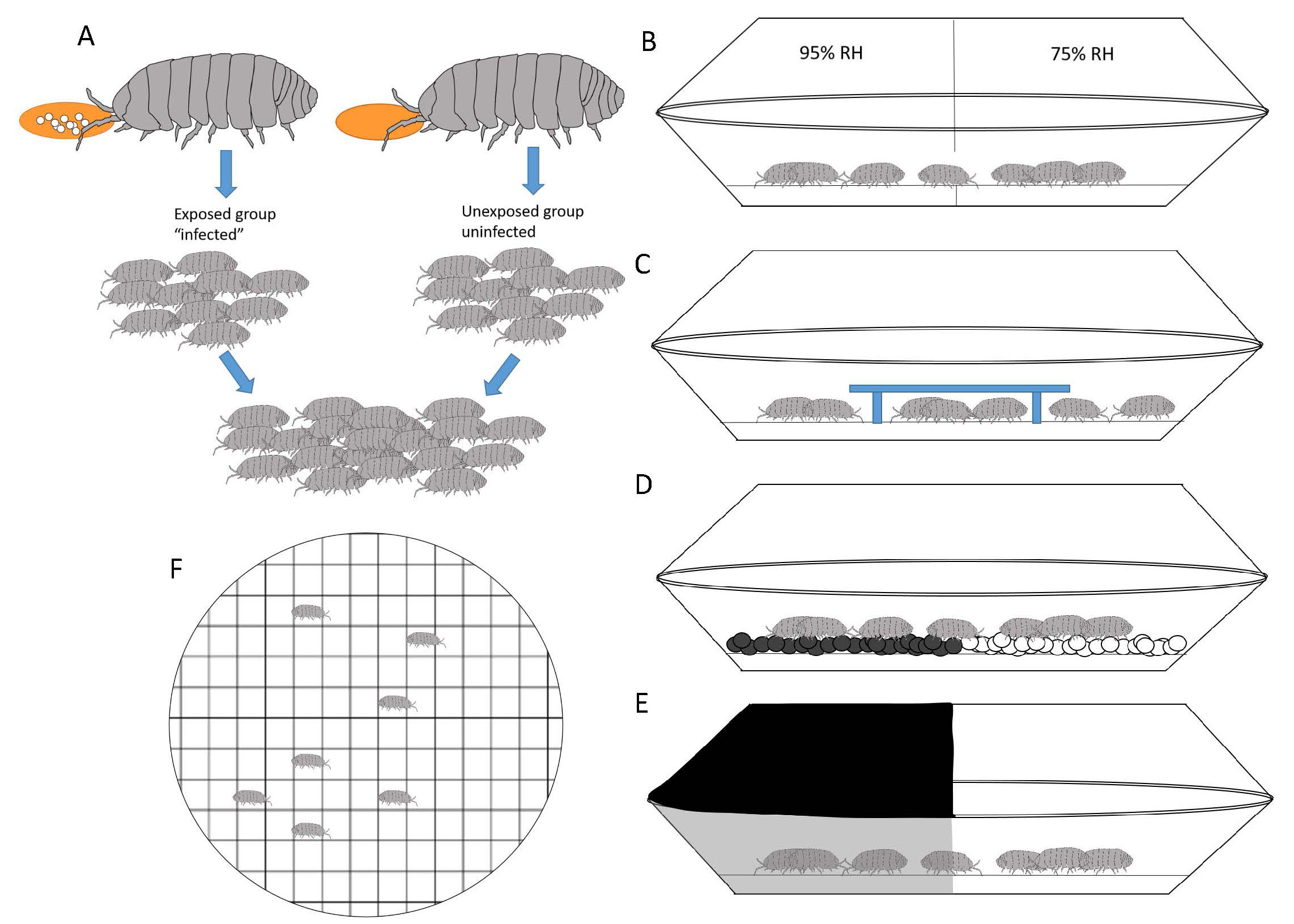
Figure 3. Experimental design to test behavioral differences between uninfected pillbugs and those infected with Plagiorhynchus cylindraceus. A) Experimental infections: Pillbugs were fed carrots with (exposed) or without (unexposed) P. cylandraceus eggs. Pillbugs were maintained for 3 months to ensure the cysticanth had reached the stage when it could be infectious to birds. Prior to placement in the arenas (B–F), an equal number of exposed and unexposed pillbugs were combined into a group, then each was uniquely marked. At the end of each trial all pillbugs were dissected to look for cysticanths. Each behavior trial was thus blind (the observer did not know infectious status during behavioral observation). All arenas consisted of 2 pie plates, one on top of the other. A wire mesh bottom was placed as a platform for pillbugs. Different aspects were manipulated to test the behavioral response. B) Humidity choice: High relative humidity (RH) or low RH. C) Shelter seeking: Under a shelter or exposed. D) Substrate preference: White or black. E) Phototaxis: Light or dark. F) Locomotion: Distance moved and resting behaviors.
(Source: Adapted from Moore, 1983. License: CC BY-NC-SA 4.0.)
Before adding the pillbugs to the arenas, 5 exposed and 5 unexposed pillbugs were mixed together and were then marked with a unique identifier. By mixing them before the study, it enabled Moore to conduct blind assays in which she did not know which pillbugs were exposed and which were unexposed. In this way she controlled for observational bias. The trials consisted of placing the 10 pillbugs in the arena, allowing them to acclimate, and then recording the location of each pillbug every minute for 30 minutes. At the end of each trial, the pillbugs were dissected to determine infection status. Moore did this for each of the different arenas: humidity choice (95% relative humidity:75% relative humidity; Figure 3B), shelter seeking (under a shelter:exposed; Figure 3C), substrate choice (white:black; Figure 3D), and phototaxis (movement to or away from light; Figure 3E).
Moore found that infected pillbugs spent significantly more time in less humid and unsheltered areas and spent more time on white substrate than uninfected pillbugs (Figure 4A-C), however there was no difference in preference for darkness (all preferred dark). Activity levels differed between infected and uninfected female pill bugs; infected females traveled further and rested less than uninfected females (Figure 4 D-E).
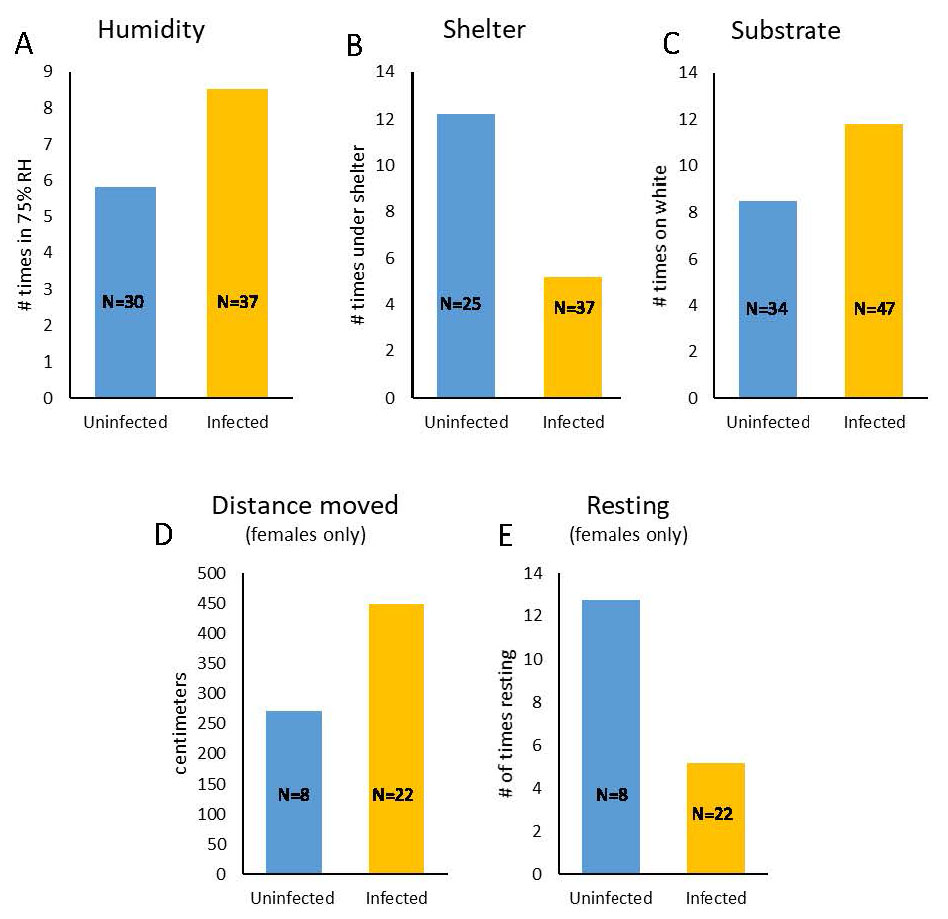
Figure 4. Infected pillbugs were found more frequently in less humid areas, in unsheltered areas, and on white substrate than uninfected pillbugs. There was no difference in phototaxis between infected and uninfected groups, both were negatively phototactic. Infected females were more active than uninfected females; infected females moved a greater distance in a set time period and rested less than did uninfected females. Males did not show differences between infection groups. The error bars are not shown (but see Moore, 1983).
(Source: Adapted from Moore, 1983. License: CC BY-NC-SA 4.0.)
The second aspect of Moore’s study was to establish whether starlings fed preferentially on infected pillbugs. Moore used experimental data from outdoor cage studies as well as observational data from the field. In the outdoor cage trials Moore used 5 individual wild-caught adult starlings and provided each individual bird with 10 infected and 10 uninfected pillbugs (pillbugs were unmarked; Figure 5). Pillbugs being presented to the birds were on a pan where they were offered a choice of black/humid or white/less-humid substrate. After the bird had eaten 10 pillbugs, the uneaten pillbugs were dissected in order to determine which pillbugs the starling had eaten (infected or uninfected). Moore found that 71% of the infected pillbugs were eaten and only 44% of the uninfected pillbugs were eaten (Figure 5), indicating that behavioral differences in the pillbugs led to an increase in predation rates on infected pillbugs.
In the field, Moore used the infection rate of nestling starlings to establish if parents were foraging randomly or preferentially on infected pillbugs. Note that nestlings can become infected by being fed infected pillbugs by their parents (Figure 2). Moore collected data from wild starlings to determine how often pillbugs were fed to nestlings and the natural infection rate of pillbugs in the field area. With these data, she calculated the probability of nestlings receiving infected pillbugs from their parents if the parents chose pill bugs randomly in the field arena. She then compared this probability to the actual infection rate of nestlings in the field. She found that more nestlings were infected than would be expected if parents were choosing pillbugs randomly. Which means that adult starlings were feeding their nestlings infected pillbugs more often than they were feeding them uninfected pillbugs because the adults are more likely to capture infected pillbugs due to the risky behavior exhibited by the infected pillbugs. These field observations corresponded with what she saw in the lab predation experiments. In conclusion, Moore provided experimental and field-based evidence that the behavioral manipulation of pillbugs by Plagiorrhynchs cylindraceus is a parasite adaptation to increase the chance of being consumed by the next host in the life cycle.
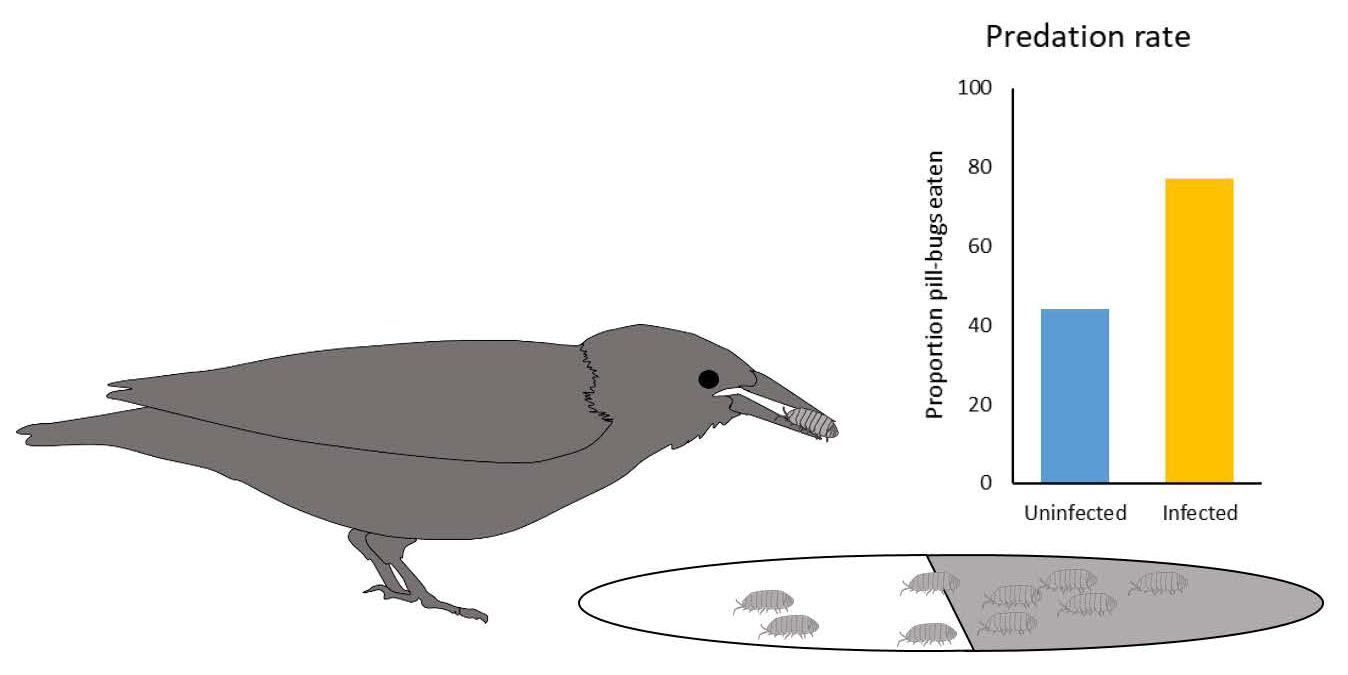
Figure 5. Field-cage predation experiment. Starlings were offered an equal number of infected and uninfected pillbugs in a pan that allowed pillbugs to choose their habitat. The habitat provided was either white dry sand or dark humid sand. Over 5 trials, 71% of infected pillbugs were eaten and only 44% of uninfected pillbugs were eaten.
(Source: Adapted from Moore, 1983. License: CC BY-NC-SA 4.0.)
Vector-Borne Transmission
Not all parasites that have a complex life cycle involve trophic transmission. Parasites that use a vector to transmit parasites to multiple hosts are also exhibiting a complex life cycle (Figure 1B), but in this case, 1 host transmits the parasite to the other without being consumed. A vector-parasite life cycle often involves an arthropod that is capable of blood-feeding (think mosquitoes, ticks, kissing bugs, sand flies) on a vertebrate host. Through the act of blood-feeding, parasites are transmitted to the other host in the life cycle, often a vertebrate.
Stop and Think
Before reading further, think about times when you have been bitten by a mosquito. You hear and see them and you likely swat or slap them or you just give up and go inside. What if that mosquito was infected with a parasite that could be transmitted to you? What mosquito behaviors might the parasite manipulate to ensure transmission to you? What behaviors might be manipulated to ensure it was also transmitted to the other people hanging around outside with you? How would you formulate these questions into hypotheses that you could test?
Behaviors that are likely to be altered in this type of host-parasite relationship are those that increase the transmission rate or the parasite load delivered upon transmission. The vector behaviors that are most often targeted are the feeding behaviors, although host-seeking/finding behaviors have also been shown to be altered by parasites (see reviews: Molyneux and Jefferies, 1986; Hurd, 2003; Lefèvre et al., 2006). One of the first accounts of modified feeding behavior of a vector was by Bacot and Martin, 1914 (referenced in Moore, 2013), where they observed that fleas carrying the plague bacterium (Yersinia pestis) were less successful at feeding due to blockage of their feeding apparatus by Y. pestis and that the blockage led to plague transmission. Plasmodium, the parasite that causes malaria, is vectored by mosquitoes and multiple studies have shown that Plasmodium can alter mosquito host-seeking and blood-feeding behavior in ways that can potentially increase transmission rates (Cator et al., 2012).
Stop and Think
How is it advantageous to the parasite to alter vector behavior only during certain times? Think about what a vector has to go through when it needs to feed? What are the risks?
Another well-studied vector-borne parasite that has been studied in light of its behavioral manipulations is the protozoan parasite Leishmania (Killick-Kendrick et al., 1977; Beach et al., 1985, citing Chung et al. 1951; Rogers et al., 2002). Leishmania are single-celled parasites that are transmitted to humans or other mammals by the bite of a sand fly and in humans can cause various debilitating pathologies and symptoms (see Chapter 12 for detailed descriptions). The most common form is cutaneous leishmaniasis which is characterized by painful open sores on the skin that have difficulty healing. Rogers and Bates (2007) investigated whether 2 species of Leishmania that cause cutaneous leishmaniasis, L. mexicana and L. infantum, manipulate the behavior of their sand fly hosts (Lutzomyia longipalpis) in ways that increase transmission efficiency in a mouse model (use of humans in experimental infections is reasonably restricted). An elegant multi-dimensional study provides evidence that Leishmania can manipulate host behavior to increase transmission and infectivity, described below (also see Table 1).
Table 1. Summary of experimental design to establish that Leishmania manipulation of sand fly feeding behavior results in enhanced transmission. [Note: The figure numbers refer to those in the cited sources.]
(Source: Adapted from Rogers and Bates (2007), 2019. License: CC BY-NC-SA 4.0.)
In order to interpret when and how behavioral alterations are likely to occur in the sand fly-Leishmania system, the life cycle of Leishmania must be understood (see Chapter 12 for more on Leishmania). In short, the life cycle of Leishmania involves an infected sand fly biting an uninfected mammalian host and injecting the motile promastigote stage. The promastigotes invade white blood cells and develop into amastigotes. An uninfected sand fly becomes infected when it bites an infected mammal and ingests blood containing the amastigote stage. In the sand fly, the amastigote stage transforms into the promastigote stage over the course of 7–10 days (extrinsic incubation period). Thus, it is important to remember that the promastigote stage is the stage that is infective to the mammal and that the amastigote stage is infective to the sand fly.
Some of the previous work on this system must be understood before delving into the study by Rogers and Bates (2007). Several research teams established that Leishmania damage the stomodeal valve and physically block the gut with a matrix made by a gel they secrete (Schlein et al., 1992; Stierhof et al., 1999; Rogers et al., 2004). This blockage interferes with sand fly feeding and limits the amount of blood it can take in. As a result, they take longer to feed and probe the skin more often (Rogers et al., 2002). A different group studying the rodent malaria-mosquito model of Plasmodium yoelli and Anopheles stephensi found that feeding persistence increased in infected mosquitoes but only after Plasmodium had reached the stage in which it was infective to humans (Anderson et al., 1999).
Armed with that background information, it can be understood how Rogers and Bates (2007) developed their hypotheses: 1) Leishmania manipulate sand flies to persist in bloodfeeding only after they become infective to mammals (when the parasite reaches the promastigote stage), 2) Leishmania-infected sand flies feed on multiple hosts, and 3) Leishmania-infected sand flies that have been behaviorally manipulated will deliver a higher parasite inoculum per host than non-manipulated infected sand flies.
In order to answer these questions, Rogers and Bates used a biting persistence assay in which individual sand flies were allowed to land and attempt feeding on an anesthetized mouse for 1 minute, after which they were disturbed by brushing the leg or antennae every 10 seconds until they stopped trying to feed (Figure 6).
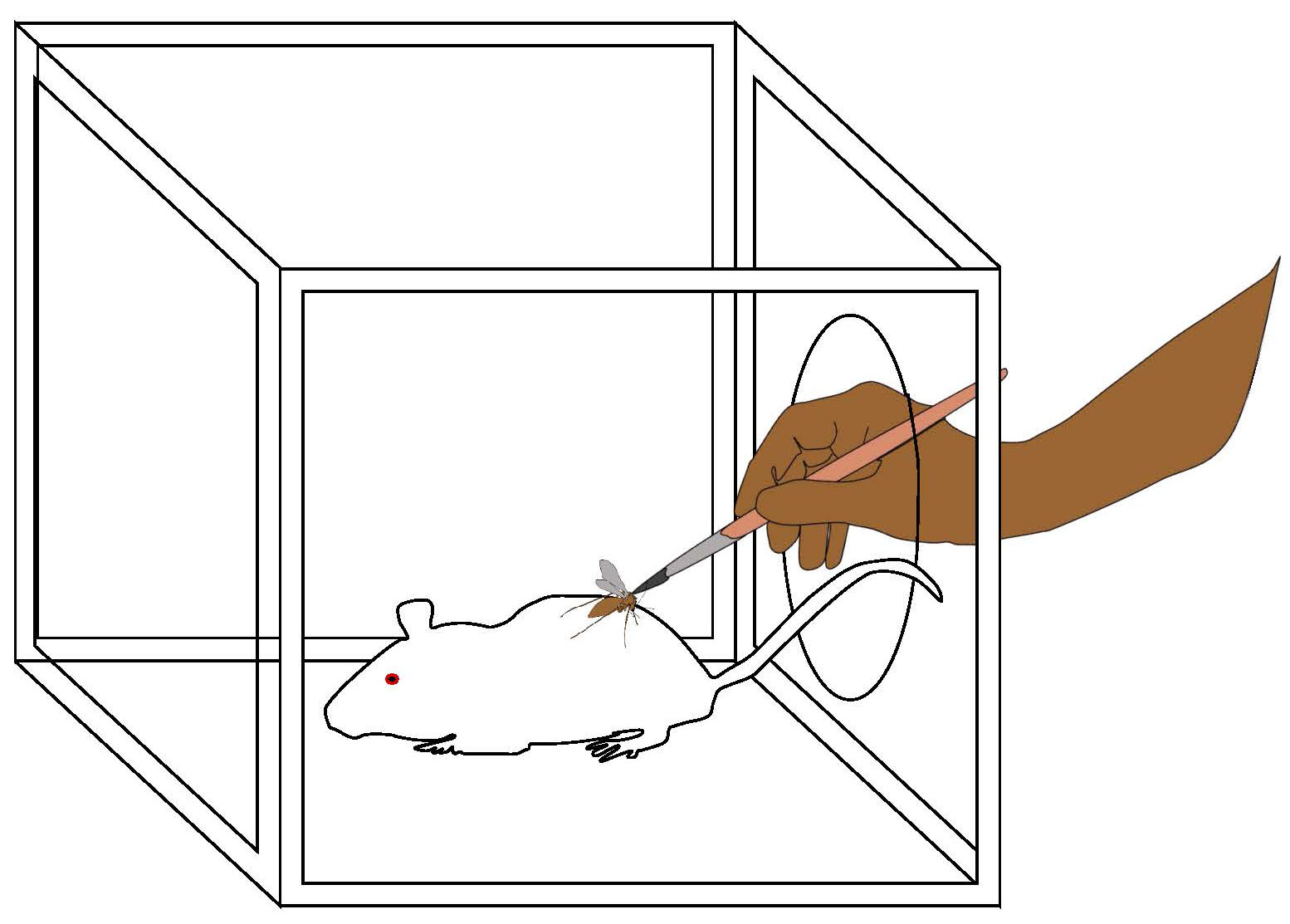
Figure 6. Biting persistence assay. One sand fly at a time was placed in a cage with a single anesthetized mouse. The sand fly was allowed to feed for 1 minute, after which it was disturbed every 10 seconds by brushing its hind legs until the sand fly stopped trying to feed. The total time it took to stop attempting to feed was known as its feeding persistence. After the trial sand flies that were experimentally infected with Leishmania were dissected and parasite load determined.
(Source: M. Wise de Valdez, 2019. License: CC BY-NC-SA 4.0.)
The time it took for the sand fly to stop attempting to feed was considered their feeding persistence. The biting assay was modified to address each of the hypotheses. In order to test the first hypothesis, Rogers and Bates experimentally infected sand flies by feeding them rabbit blood with Leishmania amastigotes (they used 2 species of Leishmania; L. mexicana and L. infantum), or rabbit blood alone (the uninfected group; Figure 7).
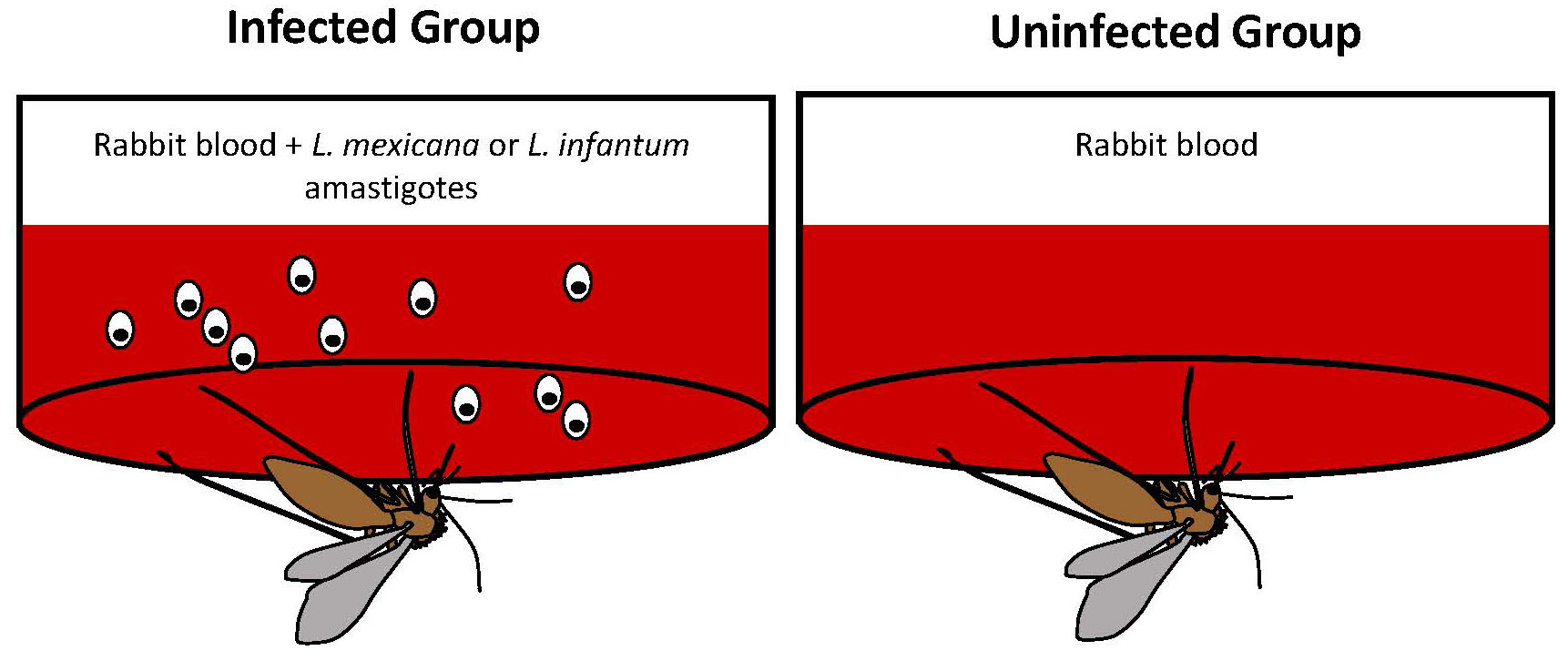
Figure 7. Experimental infection of sand flies was carried out using an artificial membrane system. Each feeder held fresh rabbit blood with either Leishmania amastigotes (L. mexicana or L. infantum) or rabbit blood alone.
(Source: M. Wise de Valdez. License: CC BY-NC-SA 4.0.)
They then used the biting persistence assay as described; testing both infected and uninfected sand flies. Recall that the first hypothesis also stated that the parasite should alter the behavior only when it becomes infective to the next host. Therefore, they conducted this assay daily over the course of the infection: Four days post-infection (non-infective stages) through 11 days post-infection (highly-infectious stages). They found that sand flies infected with either Leishmania mexicana or L. infantum exhibited greater feeding persistence than uninfected sand flies and that this occurred later in infection when the parasite could be effectively transmitted to a mammalian host (Figure 8A).
The second hypothesis, which stated that infected sand flies are more likely to feed on multiple hosts, required modifying the biting persistence assay to include a second mouse. The sand fly was allowed to locate and begin feeding on a mouse for 1 minute and then disturbed every 10 seconds until the sand fly switched to the other mouse or stopped attempting to feed. The researchers observed sand flies on days 5, 7, and 10 post-infection. They found that sand flies infected with Leishmania mexicana or L. infantum were more likely to switch to a new host than uninfected sand flies (Figure 8B). The third hypothesis required a more elaborate set-up. The hypothesis was: An increase in biting persistence leads to a greater parasite load delivered to the mammalian host. In order to test this, they had to be able to compare a group of infected sand flies that exhibited increased feeding persistence and infected sand flies that did not. Rogers and Bates were able to isolate different phenotypes of L. mexicana: One that elicited an early increase in biting persistence (7 days postinfection; exponential phase) and another that did not elicit an increase until closer to day 10 (stationary phase). They experimentally infected sand flies with either the exponential phase or the stationary phase. On day 7 post-infection, they conducted the biting persistence assay and followed the development of the resulting lesions on the mice. They used the thickness of the lesions as a proxy for the inoculum size (the number of parasites injected by the sand fly). They found that the average lesion thickness was greater in mice bitten by more persistent sand flies than less persistent sand flies (Figure 8C). In a parallel experiment to confirm that the biting persistence was the primary mechanisms for an increased inoculum, the authors allowed sand flies of both infection types to feed without interruption. They found that the average lesion thickness on mice did not differ between the 2 groups (Figure 8D). This is further evidence that the modified behavior of increased feeding persistence was the mechanism for an increase in transmission efficacy.
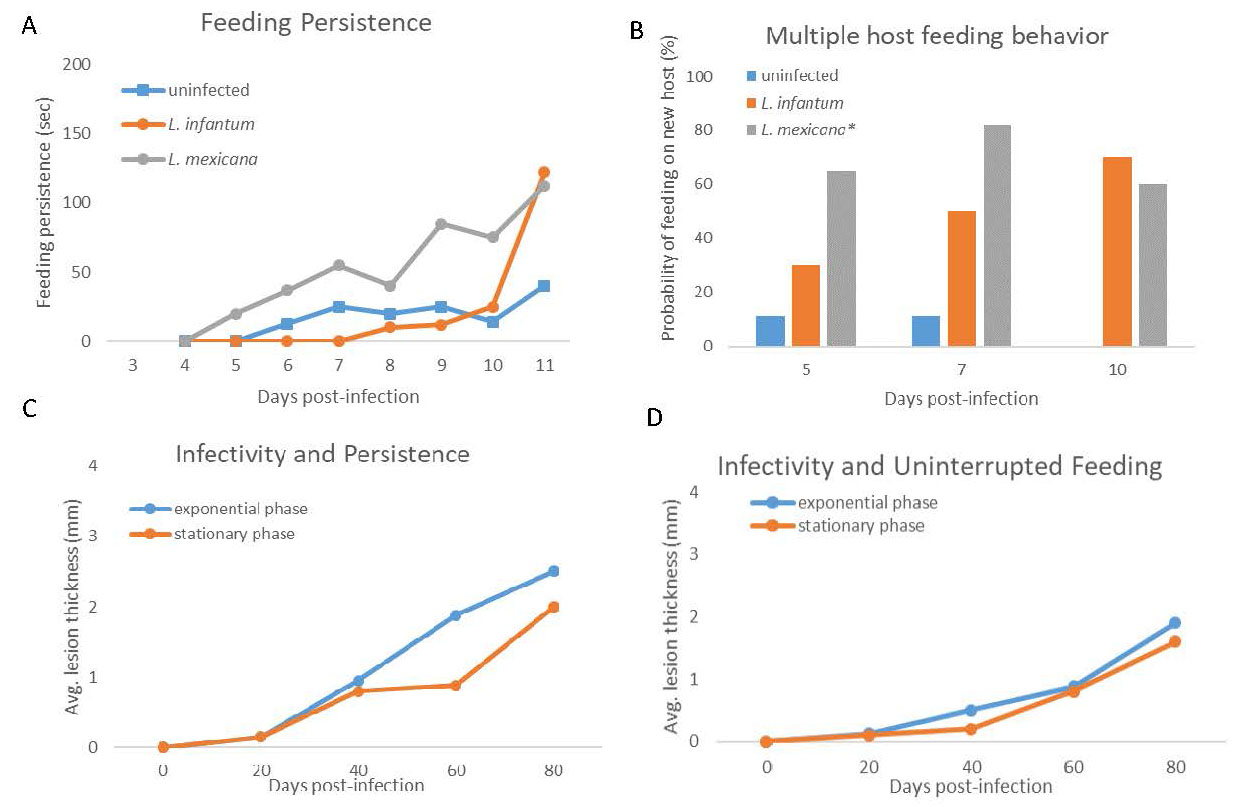
Figure 8. Results from the biting persistence assays. A) Feeding persistence of infected and uninfected sand flies. Infected sand flies exhibited greater feeding persistent than uninfected sand flies. For each day post infection, 16 infected and 16 uninfected sand flies were assayed to establish an average feeding persistence. Error bars not shown. B) Proportion of sand flies assayed that switched to a novel host after repeated interruption. On days 5, 7, and 10 post-infection 12 sand flies from each group were assayed. Error bars not shown. C) Average lesion thickness of mice bitten by persistent sand flies (blue) or non-persistent sand flies (orange). Error bars not shown. Persistent sand flies produced greater lesions and thus delivered a greater inoculum of parasite than non-persistent sand flies. D) Average lesion thickness of mice after being bitten by uninterrupted sand flies. The error bars are not shown. There was no difference in lesion thickness between the exponential and the stationary stage infected sand flies when they were allowed to feed without interruption.
(Source: Adapted from Rogers and Bates, 2007. License: CC BY-NC-SA 4.0.)
Rogers and Bates’s primary conclusions were that, 1) Timing of parasite development is linked to feeding persistence, 2) parasites do not increase risky feeding behavior until the stage that is infective, and 3) that this behavioral manipulation strategy enhances Leishmania transmission by increasing transmission to multiple hosts and increasing parasite load during biting. Thus, this set of experiments provided evidence for adaptive parasite manipulation of the vector behavior and the fact that it occurs in more than 1 species lends strength to this conclusion.
Transmission to a New Habitat
Some life cycles require that the parasites be delivered to a new habitat where they emerge themselves or where their propagules (eggs or juveniles) are released (Figure 1C). Delivery to a new habitat can be as simple as the parasite taking advantage of where its host is already going, or it may require the manipulation of a behavior to take a host where it wouldn’t normally go. Mermithid nematodes (Gastromermis) in adult mayflies (Baetis bicaudatus) do both. Gastromermis nematodes that infect mayflies use the female mayfly’s natural oviposition behavior of laying eggs in streams to reach a water source where they then emerge to mate (Vance, 1996a). However, when the nematodes find themselves in a male mayfly they are a bit stuck because males do not display oviposition behavior. Vance (1996a) showed that mermithids feminize male mayflies which causes them to exhibit oviposition behavior, thus delivering the worms to water where they can emerge. This type of study provides unique evidence that the behavioral manipulation is adaptive because the parasite does not manipulate behavior of all hosts, only those that do not exhibit the behavior necessary for it to complete its life cycle. This selectivity within the same system regarding which hosts are manipulated and which are not is indicative of a phenotype that is a direct result of natural selection. This host-parasite system is also unique because it exhibits host sex-specific manipulation.
Sometimes it is not about the adult stage emerging in a habitat where it can mate, it is also about delivering the immature stages to habitats where they can get to the next host. Plagiorchis elegans manipulates its snail intermediate host (Stagnicola elodes) to rise to the water surface to release the cercarial stage (Lowenberger and Rau, 1994) and several parasitic fungi alter the behavior of their insect host to find perching areas to better release their fungal spores (Poulin, 2010, citing Andersen et al., 2009; Maitlan, 1994).
One of the most well-known examples of parasite behavioral manipulation is horsehair worms (phylum Nematomorpha) that cause their terrestrial insect host to jump into water. Thomas and colleagues (2002) carried out field observations and experiments in the field and lab to evaluate this behavior. This study bears highlighting since it 1) includes non-manipulative field observations of multiple host species being manipulated by 2 different species of nematomorphs, and 2) the authors use a y-tube olfactometer which is a tool in studying preference and/or choice (Figure 9). Behavioral biologists across many fields use some form of the y-tube olfactometer regularly.
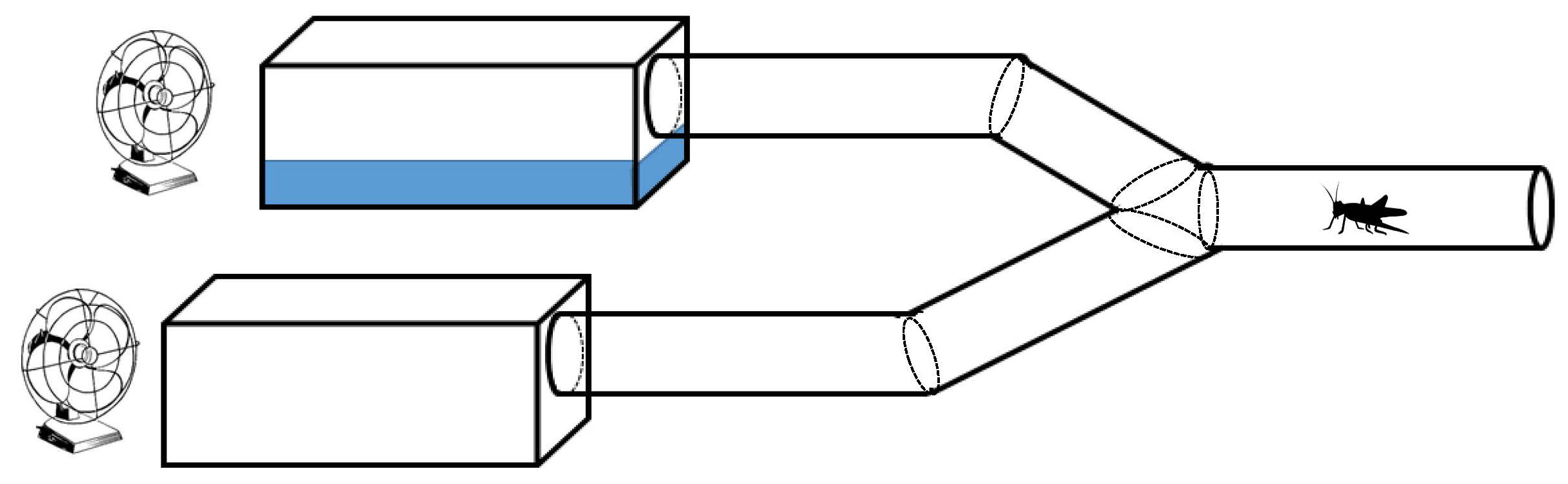
Figure 9. Y-tube olfactometer. A hypothetical design of the y-maze choice assay conducted by Thomas and others (2002) to assess whether water served as an attractive stimulus. At one end of each arm was a trough, 1 with water and 1 without. A fan was placed at the end of each arm to gently send the “odor” down each arm. Crickets were tested one at a time by placing them at the end of the tube. After 15 minutes their location was recorded.
(Source: Adapted from Thomas et al. (2002), 2019. License: CC BY-NC-SA 4.0.)
The field observations made by Thomas and colleagues (2002) involved recording the number of insects coming from a nearby forested area (with known natural habitats for nematomorphs), moving across a concrete pathway towards a swimming pool, and jumping into a pool. They also recorded how many of those insects were infected. They conducted these observations every night over 2 consecutive summers. They recorded 9 different species that jumped into the swimming pool and all were infected (Figure 10C). The most common species recorded were Nemobius sylvestris (Figure 10A), with 70 individuals that committed suicide, and Meconema thalassinum (Figure 10B), with 30 individuals taking a dip (and subqequently dying as a result).
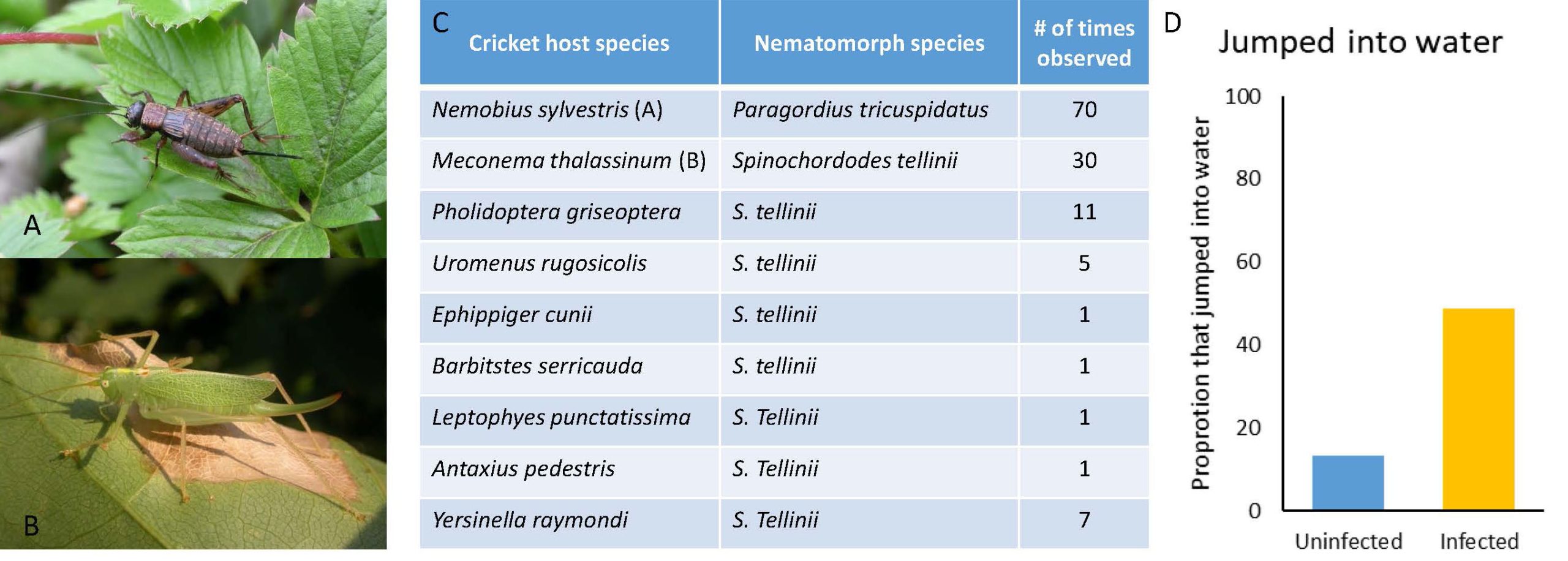
Figure 10. A) The European bush cricket Nemobius sylvestris. B) The oak-bush cricket Meconema thalassinum. C) Results of the field observations: species of crickets that jumped into water, the species of nematomorph they harbored, and the number of times they observed individuals of each species jumping into the water over the course of 2 summers. D) Results of the field experiment: Proportion of infected and uninfected crickets that jumped into the water.
(Source: Adapted from Thomas et al. (2002). License: CC BY-NC-SA 4.0.)
In the field-based experiment, Thomas and others used field-caught crickets. They collected 33 Nemobius sylvestris crickets from the forested area (presumed uninfected) and 38 from the concrete area around the pool (presumed infected). They then placed the 4 crickets, 2 from the forest and 2 from the pool, under a cup on the concrete near the pool. They studied the crickets’ behavior for 15 minutes, recording which individuals jumped into the pool. After the trial, they dissected all crickets to establish their infection status. They found that significantly more infected crickets entered the water than did uninfected crickets (Figure 10D). When they analyzed which of the 33 forest-collected crickets were infected, they found that 15% were infected, while 95% of the poolside-caught crickets were infected. This significant difference between the infection prevalence of poolside versus forest-caught crickets indicates that water-seeking behavior is more common in infected crickets.
The goal of the laboratory experiment was to determine if the presence of water was an attractive stimulus for infected crickets. They used the y-tube olfactometer (Figure 9) to allow crickets (uninfected and infected) to choose an arm with water at the end, or one without water. Again, they used field-caught crickets (forest-caught and poolside-caught). They found that infection status did not affect the arm that the crickets chose. However, of the crickets that chose the arm with water, all infected crickets jumped into the water and only 1 of the 12 uninfected crickets jumped into the water. These data clearly show that nematomorphs manipulate water-seeking behavior but the mechanism by which they alter the behavior is not via an increase in water detection.
Stop and Think
What might be some other mechanisms for how the nematomorph manipulates the behavior? How would you test this?
Hosts as a Direct Resource or Single-Host Systems
Parasite-host relationships in which the parasite has only a single host for the duration of its life cycle or which relies on the host for its own development offer a unique set of hypotheses on adaptive manipulation of host behaviors (Fritz, 1982). First, it would be expected that these parasites alter host behaviors in ways that decrease the host’s risk of predation. The parasite requires the host to stay alive long enough for the parasite to reach maturity and by altering behaviors that reduce predation on the host, the parasite thereby increases its own chance of survival. Second, it would be expected that these parasites would alter host behaviors in ways that ensure that sufficient nutritional reserves are available to the parasite. Parasites that develop to maturity in a host and then emerge usually require vast nutritional resources directly from the host. There are 2 sets of behaviors that might be targeted. Parasites might reduce energetically expensive behaviors in order to reserve nutritional stores or they might increase host foraging behavior to keep up with the nutritional needs of both the parasite and host. There are relatively few studies that experimentally investigate whether these behavioral changes occur (Moore, 2002) and fewer still that provide evidence for adaptation (but see Benton and Pritchard, 1990; Vance, 1996a; 1996b; Vance and Peckarsky, 1997; Wise de Valdez, 2007; Barquin et al., 2015; Soghigian et al., 2017).
Stop and Think
Addressed earlier was how nematomorphs manipulate their hosts to jump into water so that they can emerge. This host-parasite system is also one in which the parasite uses the nutritional stores of the insect in order to complete development and requires that the cricket host stays alive for more than a month. What other types of cricket behaviors might the nematomorph alter while it is developing?
One theme that emerges from the literature, however, is that there appears to be a trade-off between reducing predation risk and ensuring that enough nutrition is obtained. Revisiting the mermithid-nematode system helps to explain this point. Mermithid nematodes infect juvenile mayflies (in their aquatic stage) and there they undergo partial development. The mayfly larvae have to stay alive long enough to emerge into flying adults in order for the mermithid to complete its development. Therefore, it might be expected that the mermithids in the larval mayflies would reduce risky behaviors so as not to become fish food. However, they in fact increase their risky behaviors and are preyed upon more often than uninfected mayflies (Benton and Pritchard, 1990; Vance, 1996a; 1996b; Vance and Peckarsky, 1997). The researchers propose that there is a trade-off between maintaining nutritional reserves and predator avoidance. They suggest that the developing mermithid induces a nutritional deficit and therefore increasing feeding behaviors (and thus risky behaviors) may make up for that deficit. Note however, that the study has not continued past the point of establishing that a behavioral difference between infected and uninfected larval mayflies exists.
Stop and Think
What might be the next set of experiments someone would want to develop in order to test these remaining questions? Reading papers that have unanswered questions and then coming up with ideas for how someone could answer them is what budding scientists should be doing. So students should find those biological systems that have unanswered questions, or have questions yet to be asked, and find a way to answer them! (Hint: Students should talk to professors and ask if they can do research in their lab.)
In 2 larval mosquito-parasite systems researchers have been able to extend the study to answer whether behavioral changes were adaptations or not. The research by Wise de Valdez (2007) described earlier concluded that the reduction of activity levels of mosquito larvae infected with mermithid nematodes was likely not a parasite adaptation because predation rates did not decrease. Soghigian and colleagues (2017) on the other hand investigated a protozoan gregarine parasite that uses mosquito larvae as its only host. They looked at larval behavior of Aedes triseriatus infected with Ascogregarina and found that they were less active and these behavioral changes did lead to reduced predation rates by the predatory larval mosquito Toxorhynchites rutilus. This difference in results is likely due to the evolutionary relationship in these 2 systems. The latter system is common in nature where they are exposed to natural selection pressures and which presumably has a longer evolutionary relationship. The former system however used laboratory-reared colonies of the mosquito, the parasite, and the predator. Laboratory environments can shift the selection pressures these organisms face. Therefore, it is important to acknowledge and consider the source of the test organisms when interpreting the results.
In the cricket-nematomorph parasite system, Barquin and colleagues (2015) used information from several studies on the impact of insect parasitoids on the calling behavior of infected crickets compared to uninfected crickets (Cade, 1984; Zuk et al., 1993; Orozco and Bertram, 2004; Kolluru et al., 2002) to hypothesize that calling behaviors of crickets should be manipulated by nematomorphs because calling is both energetically costly and attracts the attention of auditory predators. Although this study addresses only whether behavioral alterations occur and not whether they are adaptive, this study is highlighted because it exemplifies how hosts are handled in a laboratory setting and how some behaviors need to be assessed through means other than visual observation. Next, one of the experiments conducted by Barquin and colleagues (2015) is summarized.
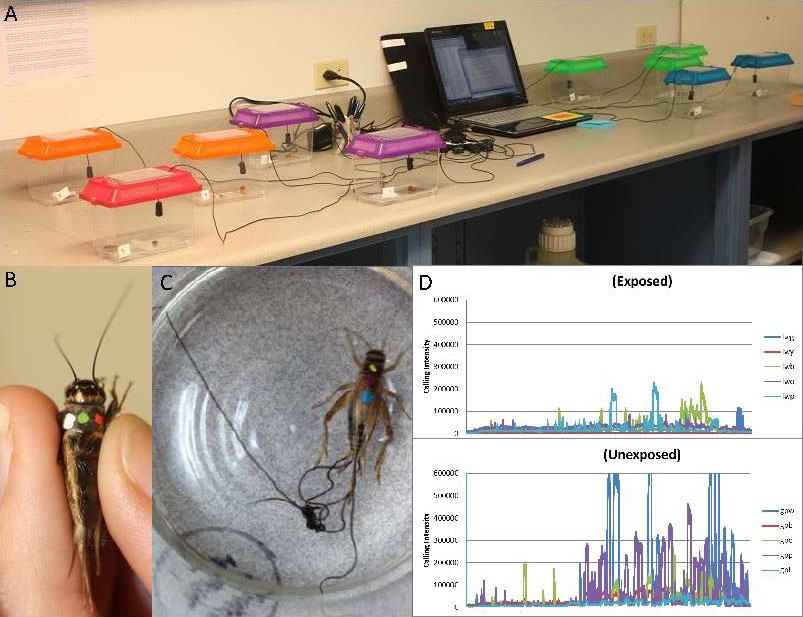
Figure 11. Experimental design used to study the effect of nematomorphs on calling behavior of crickets. A) Set up: Each cage held a microphone attached to a computer that ran a program to record frequency and intensity of calling over 12 hours. A single cricket was housed in the cage with a source of water and food. B) Example of a unique identifier. C) Sample output from a 12-hour recording period. Each different colored line was an individual cricket. Notice that on the sample day when this was recorded (6 days post-infection) the uninfected called more often and with greater frequency than exposed crickets (they did not yet know their infection status). D) Example of how the researchers checked the infection status, the nematomorph is emerging from the posterior end of the cricket.
(Source of images: M. Wise de Valdez, 2019. License: CC BY-NC-SA 4.0.)
Barquin and colleagues (2015) exposed Acheta domesticus crickets to Paragordius varius nematomorph larvae 2–3 days after wing development (30 exposed, 30 sham-exposed). Crickets were marked with waterproof paint to give them each a unique identity (Figure 11B). Crickets were housed in an insectary with a 12–12 light/dark cycle to keep the circadian rhythms. Individual cricket chirping frequency was recorded for 12 hours (dusk to dawn) on day 5 post-exposure and every 6 days thereafter using individual cages, microphones, and a computer program set up to record sound (Figure 11A).
The computer program allowed them to measure how much time they spent chirping and the intensity of the chirping events (Figure 11C). Note that the same cricket was followed throughout the course of its infection, for this reason it was imperative that each cricket had a unique identifier that would not wear off over the course of a month. After the trials infection status was determined by placing the cricket in a bowl of water and checking for worm emergence (Figure 11D). Note that exposure does not necessarily result in infection, therefore there were fewer infected crickets than uninfected crickets when data were analyzed (Figure 12).
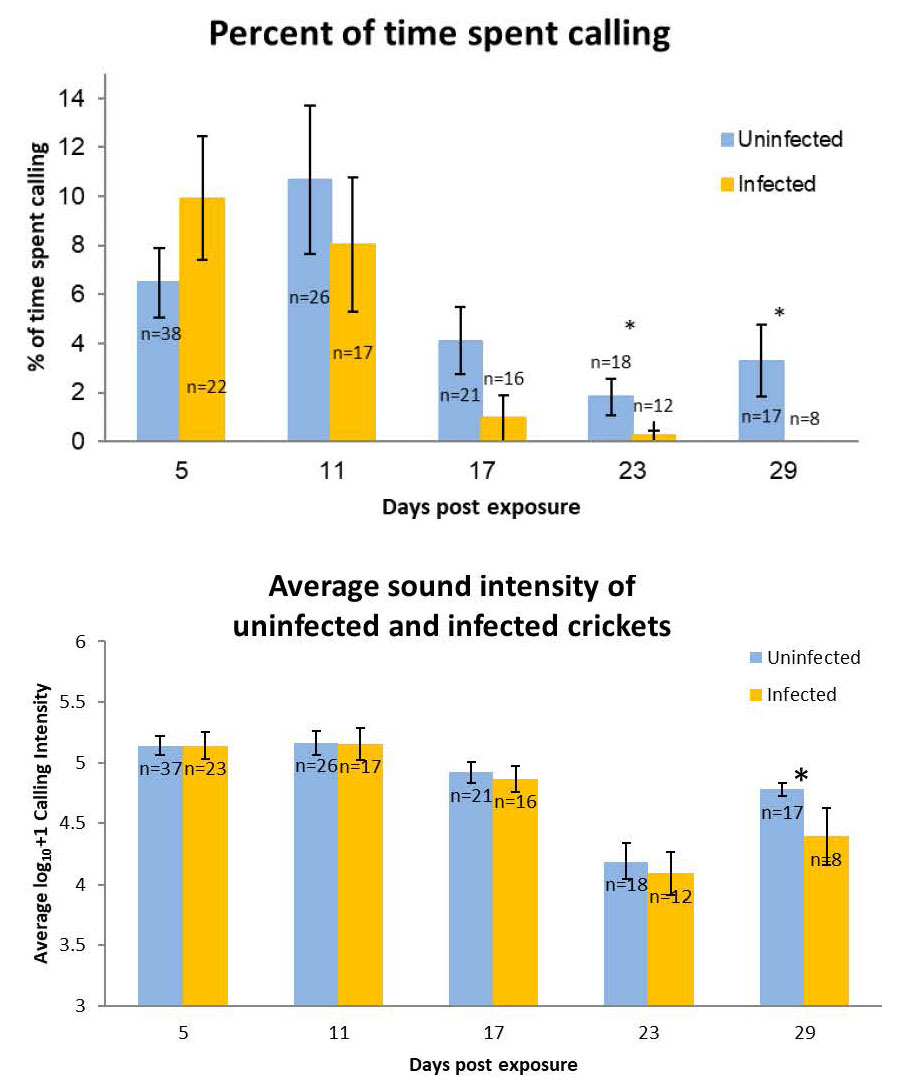
Figure 12. Time spent calling and calling intensity of male Acheta domesticus crickets infected with Paragordius varius.
(Source: Adapted from Barquin et al., 2015. License: CC BY-NC-SA 4.0.)
This section would be incomplete without mentioning that some insect parasitoids manipulate the behaviors of their hosts in ways that protect them even after they have emerged. One species of parasitic wasp manipulates its orb-weaving spider host to spin it a specialized protective pouch just before it emerges. The wasp larva is then deposited in this pouch which serves to protect it while it pupates (Poulin, 2010, citing Eberhard, 2000). Another species of parasitic wasp, which uses a caterpillar host, somehow has manipulated the caterpillar to stick around even after it emerges in order to protect it from other predators (Poulin, 2010, citing Brodeur and Vet, 1994; Grosman et al., 2008).
What Did All These Studies Have in Common?
- Started with questions and developed hypotheses that could be tested.
- The life cycle of the parasite had to be well understood.
- Needed source of infected individual: experimental infections.
- Hosts were always dissected afterwards to establish infection status.
- All studies required uninfected controls so that behaviors could be compared.
- Required both definitive and intermediate hosts as well as the appropriate habitats in the experimental design.
- Experiments were repeated: scientists used multiple organisms and multiple trials of each assay performed.
- None of them had all the answers.
A Quick Note: How Do Parasites Do It?
The mechanisms by which parasites manipulate host behaviors are elusive but more often than not they can be categorized into direct or indirect mechanisms: A direct mechanism is something produced by the parasite and an indirect mechanism might be physical interference with a biochemical pathway. Often the manipulation passes through neurological routes; some parasites secrete peptides that influence neural function, others can either directly or indirectly alter concentrations of hormones or neurotransmitters of their hosts (Poulin, 2010). A more recent area of study, proteomics, involves seeing which proteins may be manipulated by parasites and the downstream effect of those proteins on behavior (Lefèvre et al., 2009). It has also been suggested that perhaps parasites may alter the expression of host genes in a way that results in a behavioral change but this has yet to be studied (Poulin, 2010). For a more thorough discussion and concrete examples of research on how parasites manipulate behavior check out reviews by Thomas and others (2005; 2010); Lefèvre and others (2009); Poulin (2010); and Adamo (2012).
Summary/Review
Learning objectives 1, 2, and 5: Apply the scientific method to address questions about parasite manipulation of host behaviors. Analyze examples in the scientific literature to learn how scientists have experimentally addressed questions about parasite manipulation of host behaviors. Understand the types of host behaviors likely to be altered in relation to the parasites’ life cycles. The details of 4 experimental studies were described where the researchers first asked questions, formulated hypotheses, tested them, gathered and analyzed data, and interpreted the results to either support or reject their hypotheses. Each study highlighted a specific mode of transmission and the behavioral manipulations we expected to see based on those transmission modes: Trophic transmission, vector-borne transmission, transmission to a new habitat, and remaining in a host for development. Learning objective 3: Be able to provide some classic examples of parasite manipulation of host behaviors. There are 3 primary groups of parasites that always seem to be cited in the literature for behavioral parasitology: Nematomorphs, mermithid nematodes, and acanthocephalans (with a few trematodes and protozoans thrown in). Learning objective 4: Understand the evolutionary principles of parasite manipulation of host behaviors. An adaptation is any character that increases the fitness of an individual. In order for parasite-induced behavioral changes to be an adaptation they must increase the fitness of the parasite by increasing its survival so it can reproduce, increase its reproductive/transmission output, or increase its chance to make it to the next host or habitat in order to complete its life cycle. Learning objective 6: Think critically about host-parasite relationships yet to be investigated from a behavioral standpoint. Throughout the section, call out boxes urged you to stop and think. These were meant to be a pause in the reading so that you could assess whether what was being conveyed could be applied to a new scenario.
Advanced Questions
Indeed, the questions addressed throughout this section are only a few of the questions one can ask about this interesting relationship between parasites and their hosts. See also the following papers to investigate a few more relevant questions. Numerous questions are asked in several studies (Poulin, 2010; Moore, 2002; 2013; Libersat et al., 2018; Poulin and Maure, 2015; Lefèvre et al., 2009; Thomas et al., 2010; Hughes et al., 2012), such as:
- Are some taxonomic groups of parasites more likely manipulate host behavior than others?
- Why do some parasites alter behaviors and others do not?
- How effective is host manipulation?
- What behavioral changes might occur in hosts with more than 1 species of parasite?
- What other parasite-induced behavioral alterations that may benefit the host?
- How do hosts alter their behavior in order to compensate for their eventual sexual demise?
- What role might parasites that manipulate host behavior play on the ecology of the habitat in which they are found?
- What are the evolutionary mechanisms by which parasites evolve behavioral manipulation?
- What research is being conducted to determine the physical mechanism of parasite-induced altered behavior?
Literature Cited
Adamo, S. A. 1997. How parasites alter the behavior of their insect hosts. In N. E. Beckage, ed. Parasites and Pathogens. Springer, Boston, Massachusetts, United States, p. 231–245.
Adamo, S. A. 2012. The strings of the puppet master: How parasites change host behavior. In D. P. Hughes, J. Brodeur, and F. Thomas, eds. Host Manipulation by Parasites. Oxford University Press, Oxford, United Kingdom, p. 36–51.
Andersen, S. B., S. Gerritsma, K. M. Yusah, D. Mayntz, et al. 2009. The life of a dead ant: The expression of an adaptive extended phenotype. American Naturalist 174: 424–433. doi: 10.1086/603640
Anderson, R. A., J. C. Koellaf, and H. Hurd. 1999. The effect of Plasmodium yoelii nigeriensis infection on the feeding persistence of Anopheles stephensi Liston throughout the sporogonic cycle. Proceedings of the Royal Society of London B: Biological Sciences 266: 1,729–1,733. doi: 10.1098/rspb.1999.0839
Anokhin, I. A. 1966. 24-hour rhythm in ants invaded by metacercariae Dicroceolium lanceatum. Doklady Akademii Nauk SSSR 166: 757.
Bacot, A. W., and C. J. Martin. 1914. Observations on the mechanism of the transmission of plague by fleas. Journal of Hygiene (London) 13 (Supplement): 423–439.
Barquin, A., B. McGehee, R. T. Sedam, W. L. Gordy, et al. 2015. Calling behavior of male Acheta domesticus crickets infected with Paragordius varius (Nematomorpha: Gordiida). Journal of Parasitology 101: 393–397. doi: 10.1645/15-765.1
Beach, R., G. Kiilu, and J. Leeuwenburg. 1985. Modification of sand fly biting behavior by Leishmania leads to increased parasite transmission. American Journal of Tropical Medicine and Hygiene 34: 278–282. doi: 10.4269/ajtmh.1985.34.278
Benton, M. J., and G. Pritchard. 1990. Mayfly locomotory responses to endoparasitic infection and predator presence: The effects on predator encounter rate. Freshwater Biology 23: 363–371. doi: 10.1111/j.1365-2427.1990.tb00278.x
Berdoy, M., J. P. Webster, and D. W. Macdonald. 2000. Fatal attraction in rats infected with Toxoplasma gondii. Proceedings of the Royal Society of London B: Biological Sciences 267: 1,591–1,594. doi: 10.1098/rspb.2000.1182
Bethel, W. M., and J. C. Holmes. 1973. Altered evasive behavior and responses to light in amphipods harboring acanthocephalan cystacanths. Journal of Parasitology 59: 945–956. doi: 10.2307/3278623
Bethel, W. M., and J. C. Holmes. 1974. Correlation of development of altered evasive behavior in Gammarus lacustris (Amphipoda) harboring cystacanths of Polymorphus paradoxus (Acanthocephala) with the infectivity to the definitive host. Journal of Parasitology 60: 272–274. doi: 10.2307/3278463
Bethel, W. M., and J. C. Holmes. 1977. Increased vulnerability of amphipods to predation owing to altered behavior induced by larval acanthocephalans. Canadian Journal of Zoology 55: 110–115. doi: 10.1139/z77-013
Brodeur, J., and L. E. Vet. 1994. Usurpation of host behaviour by a parasitic wasp. Animal Behaviour 48: 187–192. doi: 10.1006/anbe.1994.1225
Cade, W. H. 1984. Effects of fly parasitoids on nightly calling duration in field crickets. Canadian Journal of Zoology 62: 226–228. doi: 10.1139/z84-037
Cator, L. J., P. A. Lynch, A. F. Read, and M. B. Thomas. 2012. Do malaria parasites manipulate mosquitoes? Trends in Parasitology 28: 466–470. doi: 10.1016/j.pt.2012.08.004
Chung, H. L., L. C. Feng, and S. L. Feng. 1951. Observations concerning the successful transmission of kala-azar in North China by bites of naturally infected Phlebotomus chinensis. Peking Natural History Bulletin 19: 302–326.
Cram, E. B. 1931. Developmental stages of some nematodes of the Spiruroidea parasitic in poultry and game birds. United States Department of Agriculture, Technical Bulletin, Number 227.
Eberhard, W. G. 2000. Spider manipulation by a wasp larva. Nature 406: 255–256. doi: 10.1038/35018636
Fritz, R. S. 1982. Selection for host modification by insect parasitoids. Evolution 36: 283–288. doi: 10.2307/2408046
Grosman, A. H., A. Janssen, E. F. De Brito, E. G. Cordeiro, et al. 2008. Parasitoid increases survival of its pupae by inducing hosts to fight predators. PLoS One 3: e2276. doi: 10.1371/journal.pone.0002276
Hindsbo, O. 1972. Effects of Polymorphus (Acanthocephala) on colour and behaviour of Gammarus lacustris. Nature 238: 333. doi: 10.1038/238333a0
Huffman, M. A. 1997. Current evidence for self-medication in primates: A multidisciplinary perspective. American Journal of Physical Anthropology 104: 171–200. doi: 10.1002/(SICI)1096-8644(1997)25+3.3.CO;2-K
Hughes, D. P., J. Brodeur, and F. Thomas, eds. 2012. Host Manipulation by Parasites. Oxford University Press, Oxford, United Kingdom, 224 p.
Hurd, H. 2003. Manipulation of medically important insect vectors by their parasites. Annual Review of Entomology 48: 141–161. doi: 10.1146/annurev.ento.48.091801.112722
Karban, R., and G. English-Loeb. 1997. Tachinid parasitoids affect host plant choice by caterpillars to increase caterpillar survival. Ecology 78: 603–611. doi: 10.1890/0012-9658(1997)078[0603:TPAHPC]2.0.CO;2
Killick-Kendrick, R., A. J. Leaney, P. D. Ready, and D. H. Molyneux. 1977. Leishmania in phlebotomid sandflies, IV: The transmission of Leishmania mexicana amazonensis to hamsters by the bite of experimentally infected Lutzomyia longipalpis. Proceedings of the Royal Society of London B: Biological Sciences 196: 105–115. doi: 10.1098/rspb.1977.0032
Kolluru, G. R., M. Zuk, and M. A. Chappell. 2002. Reduced reproductive effort in male field crickets infested with parasitoid fly larvae. Behavioral Ecology 13: 607–614. doi: 10.1093/beheco/13.5.607
Lefèvre, T., S. A. Adamo, D. G. Biron, D. Misse, et al. 2009. Invasion of the body snatchers: The diversity and evolution of manipulative strategies in host-parasite interactions. In J. P. Webster, ed. Advances in Parasitology 68. Academic Press, New York, New York, United States, p. 45–83. doi: 10.1016/S0065-308X(08)00603-9
Lefèvre, T., J. C. Koella, F. Renaud, H. Hurd, et al. 2006. New prospects for research on manipulation of insect vectors by pathogens. PLoS Pathogens 2: e72. doi: 10.1371/journal.ppat.0020072
Libersat, F., S. Emanuel, and M. Kaiser. 2018. Mind control: How parasites manipulate cognitive functions in their insect hosts. Frontiers in Psychology 9: 572. doi: 10.3389/fpsyg.2018.00572
Lowenberger, C. A., and M. E. Rau. 1994. Plagiorchis elegans: Emergence, longevity and infectivity of cercariae, and host behavioural modifications during cercarial emergence. Parasitology 109: 65–72. doi: 10.1017/S0031182000077775
Maitland, D. P. 1994. A parasitic fungus infecting yellow dungflies manipulates host perching behaviour. Proceedings of the Royal Society of London B: Biological Sciences 258: 187–193. doi: 10.1098/rspb.1994.0161
Molyneux, D. H., and D. Jefferies. 1986. Feeding behaviour of pathogen-infected vectors. Parasitology 92: 721–736. doi: 10.1017/S0031182000065574
Moore, J. 2013. An overview of parasite-induced behavioral alterations, and some lessons from bats. Journal of Experimental Biology 216: 11–17. doi: 10.1242/jeb.074088
Moore, J. 2002. Parasites and the Behavior of Animals. Oxford University Press on Demand, Oxford, United Kingdom, 338 p.
Moore, J. 1983. Responses of an avian predator and its isopod prey to an acanthocephalan parasite. Ecology 64: 1,000–1,015. doi: 10.2307/1937807
Müller, C. B., and P. Schmid-Hempel. 1993. Exploitation of cold temperature as defence against parasitoids in bumblebees. Nature 363: 65. doi: 10.1038/363065a0
Orozco, S., and S. M. Bertram. 2004. Parasitized male field crickets exhibit reduced trilling bout rates and durations. Ethology 110: 909–917. doi: 10.1111/j.1439-0310.2004.01022.x
Poulin, R. 1995. Evolutionary and ecological parasitology: A changing of the guard? International Journal for Parasitology 25: 861–862. doi: 10.1016/0020-7519(95)00003-k
Poulin, R. 2010. Parasite manipulation of host behavior: An update and frequently asked questions. In J. Mitani, H. J. Brockmann, T. Roper, M. Naguib, et al., eds. Advances in the Study of Behavior 41, 1st edition. Academic Press, New York, New York, United States, p. 151–186. doi: 10.1016/S0065-3454(10)41005-0
Poulin, R., and F. Maure. 2015. Host manipulation by parasites: A look back before moving forward. Trends in Parasitology 31: 563–570. doi: 10.1016/j.pt.2015.07.002
Rogers, M. E., and P. A. Bates. 2007. Leishmania manipulation of sand fly feeding behavior results in enhanced transmission. PLoS Pathogens 3: e91. doi: 10.1371/journal.ppat.0030091
Rogers, M. E., M. L. Chance, and P. A. Bates. 2002. The role of promastigote secretory gel in the origin and transmission of the infective stage of Leishmania mexicana by the sandfly Lutzomyia longipalpis. Parasitology 124: 495–507. doi: 10.1017/S0031182002001439
Rogers, M. E., T. Ilg, A. V. Nikolaev, M. A. Ferguson, et al. 2004. Transmission of cutaneous leishmaniasis by sand flies is enhanced by regurgitation of fPPG. Nature 430: 463. doi: 10.1038/nature02675
Schlein, Y., R. L. Jacobson, and G. Messer. 1992. Leishmania infections damage the feeding mechanism of the sandfly vector and implement parasite transmission by bite. Proceedings of the National Academy of Sciences of the United States of America 89: 9,944–9,948. doi: 10.1016/S0169-4758(10)80001-8
Soghigian, J., L. R. Valsdottir, and T. P. Livdahl. 2017. A parasite’s modification of host behavior reduces predation on its host. Ecology and Evolution 7: 1,453–1,461. doi: 10.1002/ece3.2748
Stierhof, Y. D., P. A. Bates, R. L. Jacobson, M. E. Rogers, et al. 1999. Filamentous proteophosphoglycan secreted by Leishmania promastigotes forms gel-like three-dimensional networks that obstruct the digestive tract of infected sandfly vectors. European Journal of Cell Biology 78: 675–689. doi: 10.1016/S0171-9335(99)80036-3
Thomas, F., S. Adamo, and J. Moore. 2005. Parasitic manipulation: Where are we and where should we go? Behavioural Processes 68: 185–199. doi: 10.1016/j.beproc.2004.06.010
Thomas, F., R. Poulin, and J. Brodeur. 2010. Host manipulation by parasites: A multidimensional phenomenon. Oikos 119: 1,217–1,223. doi: 10.1111/j.1600-0706.2009.18077.x
Thomas, F., A. Schmidt‐Rhaesa, G. Martin, C. Manu, et al. 2002. Do hairworms (Nematomorpha) manipulate the water seeking behaviour of their terrestrial hosts? Journal of Evolutionary Biology 15: 356–361. doi: 10.1046/j.1420-9101.2002.00410.x
Vance, S. A. 1996a. The effect of the mermithid parasite Gasteromermis sp. (Nematoda: Mermithidae) on the drift behaviour of its mayfly host, Baetis bicaudatus (Ephemeroptera: Baetidae): A trade-off between avoiding predators and locating food. Canadian Journal of Zoology 74: 1,907–1,913. doi: 10.1139/z96-215
Vance, S. A. 1996b. Morphological and behavioural sex reversal in mermithid–infected mayflies. Proceedings of the Royal Society of London B: Biological Sciences 263: 907–912. doi: 10.1098/rspb.1996.0134
Vance, S. A., and B. L. Peckarsky. 1997. The effect of mermithid parasitism on predation of nymphal Baetis bicaudatus (Ephemeroptera) by invertebrates. Oecologia 110: 147–152. doi: 10.1007/s004420050143
Van Dobben, W. 1952. The food of the cormorant in the Netherlands. Ardea 40: 1–63.
Watson, D. W., B. A. Mullens, and J. J. Petersen. 1993. Behavioral fever response of Musca domestica (Diptera: Muscidae) to infection by Entomophthora muscae (Zygomycetes: Entomophthorales). Journal of Invertebrate Pathology 61: 10–16. doi: 10.1006/jipa.1993.1003
Wise de Valdez, M. R. 2006. Parasitoid-induced behavioral alterations of Aedes aegypti mosquito larvae infected with mermithid nematodes (Nematoda: Mermithidae). Journal of Vector Ecology 31: 344–354. doi: 0.3376/1081-1710(2006)31[344:PBAOAA]2.0.CO;2
Wise de Valdez, M. R. 2007. Predator avoidance behavior of Aedes aegypti mosquito larvae infected with mermithid nematodes (Nematoda: Mermithidae). Journal of Vector Ecology 32: 150–153. doi: 10.3376/1081-1710(2007)32[150:PABOAA]2.0.CO;2
Yanoviak, S. P., M. Kaspari, R. Dudley, and G. Poinar, Jr. 2008. Parasite-induced fruit mimicry in a tropical canopy ant. American Naturalist 171: 536–544. doi: 10.1086/528968
Zuk, M., L. W. Simmons, and L. Cupp. 1993. Calling characteristics of parasitized and unparasitized populations of the field cricket Teleogryllus oceanicus. Behavioral Ecology and Sociobiology 33: 339–343. doi: 10.1007/BF00172933
Supplemental Reading
Hughes, D. P., and F. Libersat. Parasite manipulation of host behavior. Current Biology Magazine 29: R45–R47. https://www.cell.com/current-biology/pdf/S0960-9822(18)31602-6.pdf
Poulin, R. 2013. Parasite manipulation of host personality and behavioural syndromes. Journal of Experimental Biology 216: 18–26. doi: 10.1242/jeb.073353
Noun
From Latin: ad = near; aptus = fit
Definition: The process and condition of showing fitness for a particular environment, as applied to characteristics of a structure, function, or entire organism; also the process by which such fitness is acquired
Definition: The process of elimination of the least fitted individuals, and hence species, by the natural conditions of their habitat
Definition: One which alternates with the definitive host in which the parasite passes through partial development, but not to sexual maturity
Definition: Host in which the terminal (frequently sexual) stage of the parasite occurs
Synonym: Primary host

