8 Distributional Ecology of Parasites
A. Townsend Peterson
Introduction
Organisms in general experience an array of factors in shaping their geographic distributions. These factors are studied in the field that is coming to be called distributional ecology and range from spatial to environmental and historical to current; as such, the complexity of the situation is quite impressive. The field of distributional ecology is simultaneously pretty old (Grinnell, 1914; 1917a; 1917b) and quite new and novel (Soberón and Nakamura, 2009; Peterson et al., 2011)—distributional ecology centers around the question of why populations of a species are where they are, and why they are not where they are not. These ideas became popular with the development of large-scale and openly accessible data resources (Peterson et al., 2016), and of sophisticated computational algorithms for relating known occurrences of species to raster (that is, grid-based) GIS datasets to discover dimensions ostensibly of the fundamental ecological niche (Escobar and Craft, 2016). This old-and-new field has now seen intensive research attention from across the fields of ecology, biogeography, and systematics, and even fields as far afield as public health, invasion biology, and agricultural planning (for example, Mainali et al., 2015; Reddy and Nyári, 2015; Samy et al., 2016; Ramírez-Gil et al., 2019).
Parasites, of course, present several additional levels and dimensions of complexity for distributional ecology. The distributions of many free-living organisms (for example, plants, birds, fish) were hypothesized originally by Grinnell (1917b) to be shaped primarily by abiotic factors (for example, temperature, soil pH, precipitation; the important point is that these factors are unaffected by the presence of the species in question). However, parasites often have additional constraints. In particular, Hutchinson (1957) outlined a more complex and comprehensive niche theory that included both abiotic and biotic dimensions—these latter biotic dimensions may or may not be important in shaping geographic-scale distributions of species (Anderson, 2017). As a consequence, Peterson and others (2011) proposed the Eltonian Noise Hypothesis, the proposition that biotic interactions do not frequently constrain geographic-scale distributions of species (Peterson et al., 2011). This hypothesis—to the extent that it holds true—allows researchers to focus on ecological niches in terms of abiotic factors solely (Peterson et al., 2011). Of course, parasite distributions may be much more complicated in that biotic interactions are at times absolute: Some parasites may be incapable of surviving without specific host species being present. In sum, careful thinking about the distributional ecology of parasites will involve more complexity than is required for free-living organisms (Peterson, 2008; 2014; Escobar and Craft, 2016).
This chapter will provide a review of conceptual bases for distributional ecology. However, distributional ecology is a broad area of inquiry, such that a full and exhaustive review of the field would be too lengthy. As such, in this chapter, the focus is on what is presently perhaps the most popular methodology—that of correlative ecological niche modeling—in the parasitology literature over the past couple of decades. Still, without a doubt, other approaches and ideas should also be brought to bear on these questions, as insights based on multiple, complementary sets of analyses from distinct perspectives will generally be more robust and more likely to prove true in the long run.
Conceptual Framework
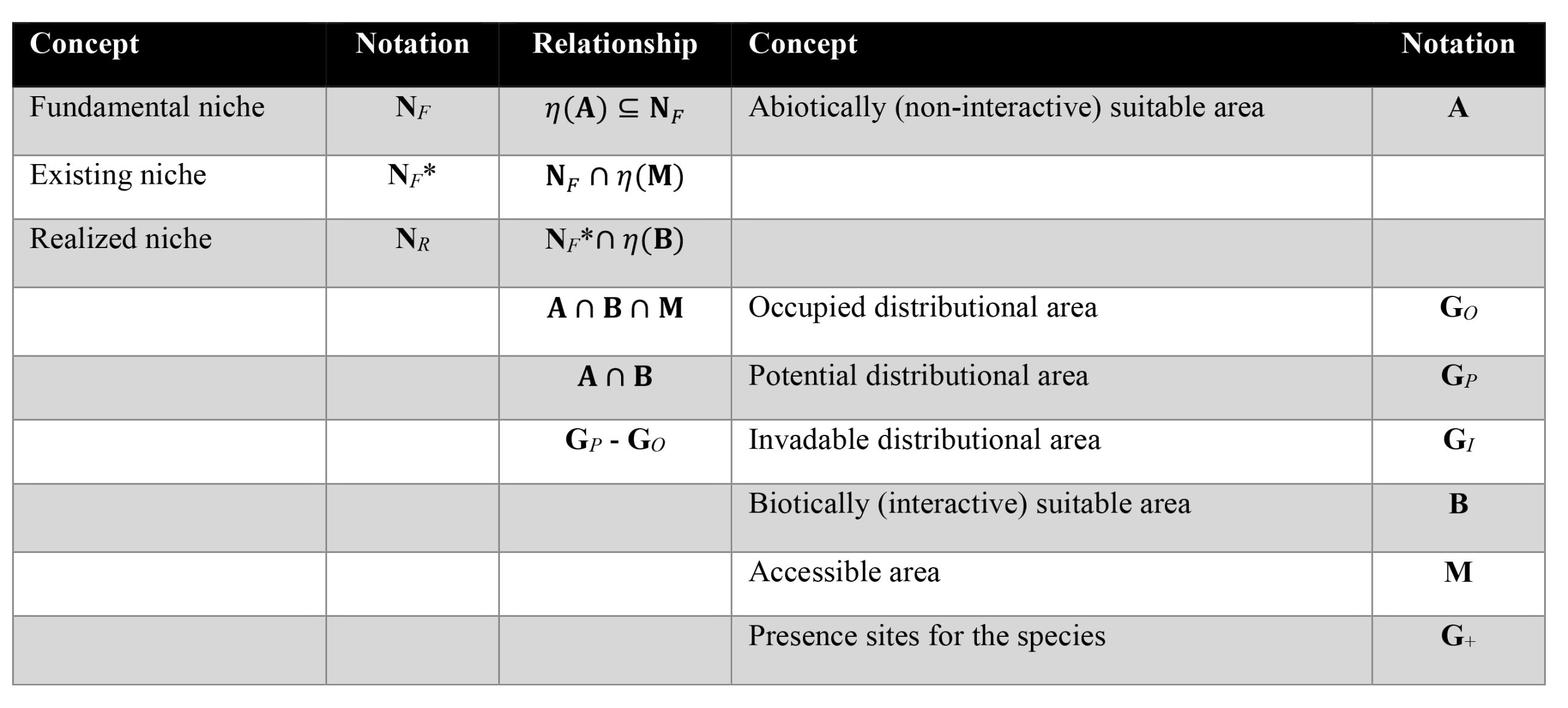
Early thinking about parasite distributional ecology was laid out by Pavlovsky (1966), who posited that foci (‘nidi’) of pathogen transmission are driven by interactions among various components of ecosystems. However, a genuinely synthetic understanding is still lacking (it is also lacking more generally for free-living, non-parasitic organisms, by the way!). That is to say that, yes, several concepts are well-known: The fundamental ecological niche, which represents an upscaling of organismal environmental physiology, and relates the persistence or fitness of a population or set of populations to a particular set of environmental conditions (Peterson et al., 2011). The fundamental ecological niche can be modified by biotic interactions to yield the realized ecological niche (Hutchinson, 1957); most treatments have assumed that these interactions are negative (for example, competition, parasitism, predation), but positive interactions can also exist. These various niches translate into the geographic distribution of the population or species, but in non-specific and non-linear ways, thanks to the complexities of the relationships between geographic and environmental spaces, which has been termed the Hutchinsonian Duality (Colwell and Rangel, 2009).
Early contributions in distributional ecology included the concept of an ecological niche that is defined in terms of physical characteristics of the environment (Grinnell, 1917a; 1917b), which has been termed the Grinnellian niche, and is roughly equivalent to a fundamental ecological niche defined only in abiotic (non-interactive) dimensions. Later came the idea of the niche being defined in multidimensional spaces and the contrasting ideas of fundamental and realized niches (Hutchinson, 1957). Perhaps least famous but most important is the idea of the existing niche as the subset of the fundamental niche that is manifested on regions that have been accessible to the species (known in previous literature as potential niche; Pulliam, 2000). Although different terminologies do exist (Sillero, 2011), the focus here is on what is probably the most comprehensive theoretical framework in distributional ecology as regards ecological niches of species (Soberón and Peterson, 2005; Soberón and Nakamura, 2009; Peterson et al., 2011).
Grinnell (1917b) developed his niche ideas in terms of tolerances with respect to physical characteristics of the environment, so these environmental dimensions are now called Grinnellian environmental variables (Tingley et al., 2009). In modern terminology, those physical characteristics are termed non-interactive variables, as they are independent of the presence of the species in question: The presence or absence or high or low abundance of the species in question does not affect Grinnellian variables, such as annual mean temperature (Peterson et al., 2011). Hutchinson (1957) introduced the idea of biotic interactions as a modifying factor in distributional ecology—these biotic factors (for example, presence of prey or a host, absence of a predator, absence of a pathogen) are now known as interactive variables (Peterson et al., 2011), and are those that are affected by the presence of the species in question, as direct feedbacks exist between abundance of the species of interest and these variables—for example, prey density.
The environments manifested across the suite of geographic sites that are within the species’ fundamental ecological niche are referred to as the existing niche, which is the set of conditions that the species has explored and tested, and where the species could potentially establish populations. Given the challenges of understanding where a species could potentially maintain populations, compared to where it actually is present, Soberón and Peterson (2005) emphasized the idea that geographic distributions are limited not just by niche considerations, but also by dispersal ability and access, such that they proposed the so-called BAM framework. According to the BAM framework, the occupied geographic distribution of a species represents the 3-way intersection of the areas suitable with respect to interactive variables (B for biotic), areas suitable with respect to non-interactive variables (A for abiotic), and areas accessible to the species over relevant periods of time (M for mobility).
Species, however, are distributed simultaneously in 2 linked spaces: The BAM diagram is cast in geographic dimensions, whereas niches are manifested in environmental dimensions. As referred to above, this dual-space nature of distributions of species is referred to as the Hutchinsonian Duality (Colwell and Rangel, 2009), which is the complex and non-linear set of connections between geographic and environmental spaces, and the idea that the species must maintain a non-null distribution in both spaces continuously and simultaneously. This concept leads to the discussion of distributions of species in environmental dimensions as different sorts of niches and distributions of those same species in geographic dimensions as geographic distributional areas. The fundamental niche represents that set of environmental conditions (in non-interactive dimensions) within which the species can maintain populations without immigrational subsidy. The intersection of the fundamental niche with the set of environments represented across M (the area accessible to the species over relevant time periods) is termed the existing niche (equivalent to the putative potential niche of Pulliam, 2000), and the reduction of the existing niche by the set of environments that are suitable for the species in interactive (biotic) dimensions is the realized niche (Peterson et al., 2011). These ideas are presented diagrammatically in Figure 1, as is the idea that the biotic influences themselves reflect BAM-type interactions of each interacting species.
In sum, the above is a brief, text-based summary of major concepts in distributional ecology. In effect, in hand, is a taxonomy of distributional areas and ecological niches, such that one can be explicit and clear in discussing and describing distributional phenomena. It is not enough to say, “I am developing a niche model” or “I am developing a distribution model” (see title of Godsoe, 2010: “I can’t define the niche but I know it when I see it …”), because the question then has to be asked as to which niche or which distribution is the object of modeling. Rather, if distributional ecology is to be a rigorous area of inquiry, explicit terminology becomes crucial; the above description is an attempt to provide such a framework for such a terminology (see Table 1 for detailed definitions of each of these concepts).
Relevant Questions in Distributional Ecology
Hutchinson’s Duality indicates that the field of distributional ecology can (and indeed must) explore both geographic and environmental dimensions of distributions of species. That is, on one side, questions are feasibly addressed that have to do with geographic distributions. For example, what is the full geographic distribution of a parasite and what host species likely remain to be discovered and documented? If closely related species tend to share the same fundamental ecological niche (Peterson et al., 1999; Peterson, 2011), then these techniques can also be used to make predictions regarding the location of undescribed species (Raxworthy et al., 2003; Peterson and Navarro-Sigüenza, 2009). Similarly, if fundamental ecological niches remain stable across time and if one has raster data layers that describe environmental conditions both at present and in the future or past, one can assess or anticipate future or past potential distributional patterns of the species.
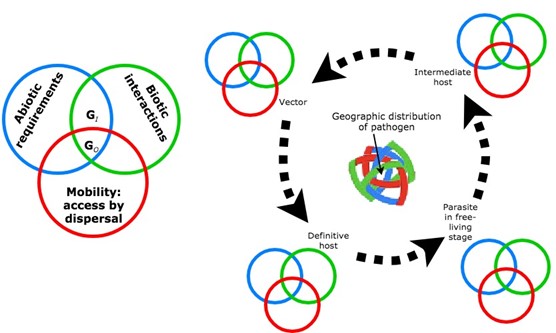
Figure 1. Summary of basic principles of distributional ecology, adapted to parasite biology. Specifically, at the left is the BAM diagram, a heuristic useful for conceiving of a species’ geographic distribution as the geographic area that (1) fits the species’ abiotic requirements (blue circle), (2) includes all necessary biotic conditions (green circle), and (3) is accessible to the species via dispersal (red circle). At the right is a hypothetical parasite life cycle, in which a parasite passes through a free-living stage, and subsequently infects an intermediate host, and is passed by a vector to a definitive host. Each of these steps in the cycle involves a set of interactions with abiotic and biotic environments, and access to a restricted set of areas (that is, a BAM intersection for each species in the parasite cycle), such that the 4-way interaction shown in the center of the life cycle would be a hypothesis of the possible geographic distribution of the parasite.
(Source: A. T. Peterson, 2019. License: CC BY-NC-SA 4.0.)
Table 1. Summary of concepts and ideas relevant to species’ geographic and environmental distributions. Note that the operator η(X) indicates the set of environments associated with some area X in geographic space.
On the environmental side, one can feasibly explore the suites of conditions associated with the distribution of a species, interpreting those conditions as manifestations of the species’ realized ecological niche. For parasites in particular, questions of realized versus existing niches emerge, as the degree to which a parasite’s range is a function of its own requirements versus those of its host(s) is a critical question in distributional ecology (Maher et al., 2010). Ideally, a deep and detailed understanding of the various niches of a species (that is, realized, existing, fundamental) should permit a predictive understanding of its distribution in time and space, and in relation to other species, including parasites, vectors, hosts, and other competitor parasites. Of particular interest is the opportunity to estimate the fundamental niche, as a fundamental niche represents an evolved characteristic of a species and should be able to be transferred to diverse sets of environmental conditions to hypothesize distributional potential.
Methodology and Study Design
Ecological niche modeling requires 2 major data inputs, and a number of decisions regarding strategy and parameter values (see Figure 2 for a diagrammatic summary, and book-length methodological summaries: Franklin, 2010; Peterson et al., 2011; Peterson, 2014; Guisan et al., 2017). The first data input is that of species occurrence data—that is, geographic coordinate pairs that correspond to locations where the species is known to have occurred. Of course, these data need to be explored, and erroneous or inconsistent records need to be detected and removed (Chapman, 2005; Cobos et al., 2018); frequently, geographic coordinates and associated uncertainty measures and documentary metadata need to be added to the data records (Chapman and Wieczorek, 2006). Finally, the occurrence data must be inspected for areas of overly intense sampling, duplicate records, or imprecise records, to avoid introducing biases.
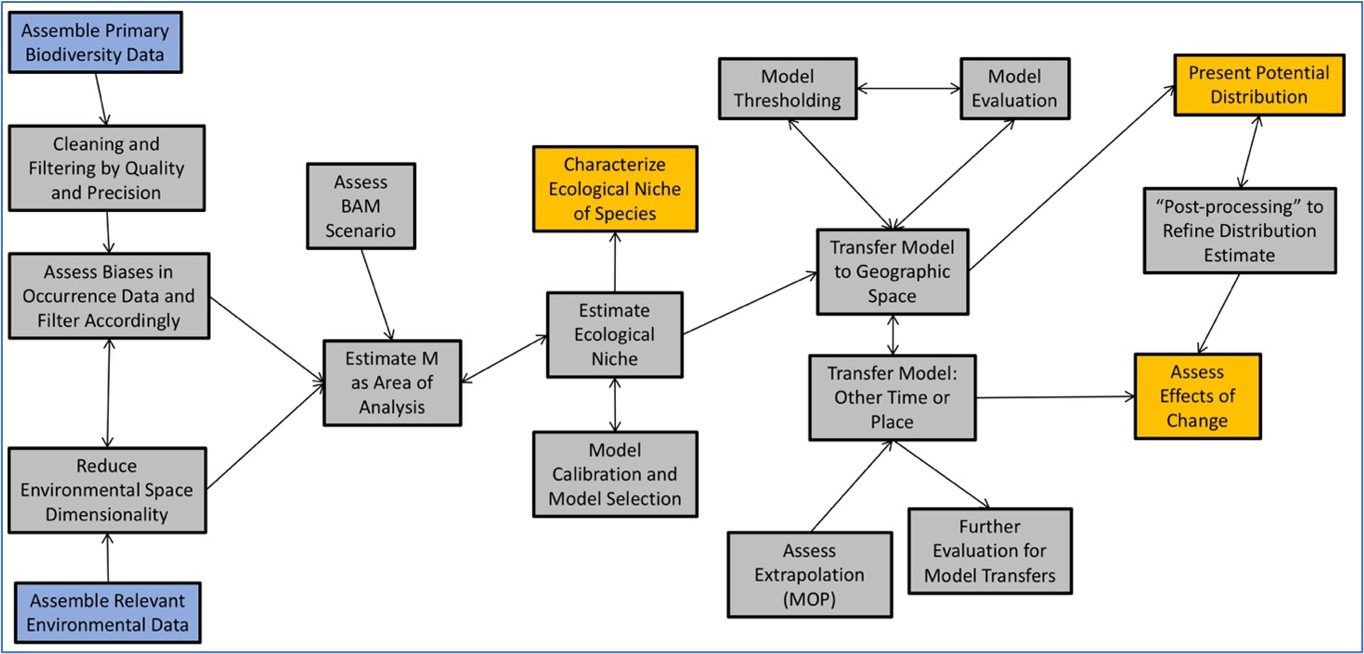
Figure 2. General summary of flow of work, inputs, and products, in ecological niche modeling. Blue boxes indicate data inputs, gray boxes are steps in the process, and gold boxes are outputs. Arrows direction denotes the flow of information.
(Source: A. T. Peterson, 2019. License: CC BY-NC-SA 4.0.)
The other major data input is that of environmental data, in the form of raster GIS data layers. Most niche-modeling algorithms require that these data layers have the same grid system (that is, spatial resolution, origin, and orientation), and indeed most studies have centered on a single climate summary (Hijmans et al., 2005), but one must think more deeply than just that. Rather, in ecological niche modeling, the modeler does not have much freedom to explore massive numbers of environmental dimensions because of problems with model overfitting in too-highly-dimensional environments (Peterson, 2007), so modelers must choose carefully the most interesting or relevant dimensions associated with the persistence of populations of a species. Of course, one approach is simply to “let the data choose,” and use the niche modeling algorithm as a sort of data-mining algorithm, but generally a better approach is to assess what is known of the species’ natural history, and to pick environmental data layers accordingly.
Once the data streams are identified and prepared, then the niche modeler must begin to integrate them. A first step is that of estimating the accessible area M, which ends up being the key area over which models should appropriately be calibrated (Barve et al., 2011). A further step is that of assessing or approximating the relative configuration of the BAM diagram for that particular species in that particular situation, because certain BAM configurations invariably lead to bad models that have little or no predictive power (Saupe et al., 2012; Qiao et al., 2015). A few adjustments can be made, though some situations simply are not appropriate for modeling.
Actual niche model calibration is accomplished by means of various algorithms (see illustrations in Figure 3). The algorithms range from the simplest, BIOCLIM, which is an approach to delineating niche estimates as orthogonal tolerance limits in different dimensions based on observed ranges of values, to complex multivariate statistical and machine-learning approaches. Each of this diversity of approaches to estimating niches has its own complexities about how it can and should be calibrated and executed (Muscarella et al., 2014; Sánchez-Tapia et al., 2017). At the end of the model calibration process, the model is generally evaluated via some sort of test of its ability to predict independent data sets, usually in geographic space. These tests can be threshold-dependent or threshold-independent, but all devolve into testing how well the model anticipates the independent occurrence data sets in the smallest area possible (Fielding and Bell, 1997). Once models are calibrated and evaluated, they can be interpreted, or transferred to other times or other regions.
A Worked Example
Here, as an example of the concepts described above, and a bit of an illustration of the inferences that can and cannot be derived from ecological niche modeling of parasites. The wasp Vespula austriaca is analyzed as an obligate parasite of its congener V. rufa (Taylor, 1939). Occurrence data were gathered for the 2 species from the Global Biodiversity Information Facility (February 28, 2019; queries are available at doi: 10.15468/dl.blijyg and doi: 10.15468/dl.w6spai), and reduced their coverage to western Europe, where point densities were greatest, as a proxy of areas where the species have established successful populations. Figure 3 presents visualizations of the distribution of the 2 species in geographic and environmental spaces.
A first consideration is that of how to characterize the fundamental niches of the species, and many methodological options are available. Focusing for the moment on Vespula rufa, the host species, one of the classic approaches to ecological niche modeling is the so-called BIOCLIM approach (Nix, 1986), which basically consists of defining tolerance limits independently in each environmental dimension, creating a multidimensional parallelepiped (Figure 4). This area nicely incorporates all (or nearly all) of the records of the species, but it also tends to include too much environmental space. More modern methods, however, such as Maxent, boosted regression trees, random forests, and general additive models, tend to be more complex in the response types that they reconstruct, which has been seen as an advantage (shown diagrammatically in Figure 4; Elith et al., 2006). However, an emerging realization is that such highly complex reconstructions of response types may not be particularly biologically realistic, as theory and experimental results from physiological studies suggest that fundamental niches should be relatively simple, and effectively convex in environmental space (Maguire, 1973). As such, a more appropriate model of a fundamental niche might enforce the simple and convex nature of these niches (see Figure 4, ellipsoid model).
A final point regards the parasite and its distribution. Several studies in the literature indicate that Vespula austriaca is an obligate parasite that focuses on V. rufa across its European distributional area. This idea is borne out by the co-distribution of the 2 species, such that no sites are apparent where V. austriaca exists in areas where V. rufa is not at least close by (Figure 3). As such, one can take the environmental distribution of the host as defining the biotically suitable area B for the parasite; the final panel of Figure 4 shows the environmental distributions of the 2 species together and points out some possible niche limitation of V. austriaca even within the bounds set by the ecological niche of V. rufa. Note that the niche of the parasite remains undefined on 2 sides simply because sites presenting environments in those directions are either 1) not accessible to the parasite or 2) not within the niche of the parasite’s obligate host.
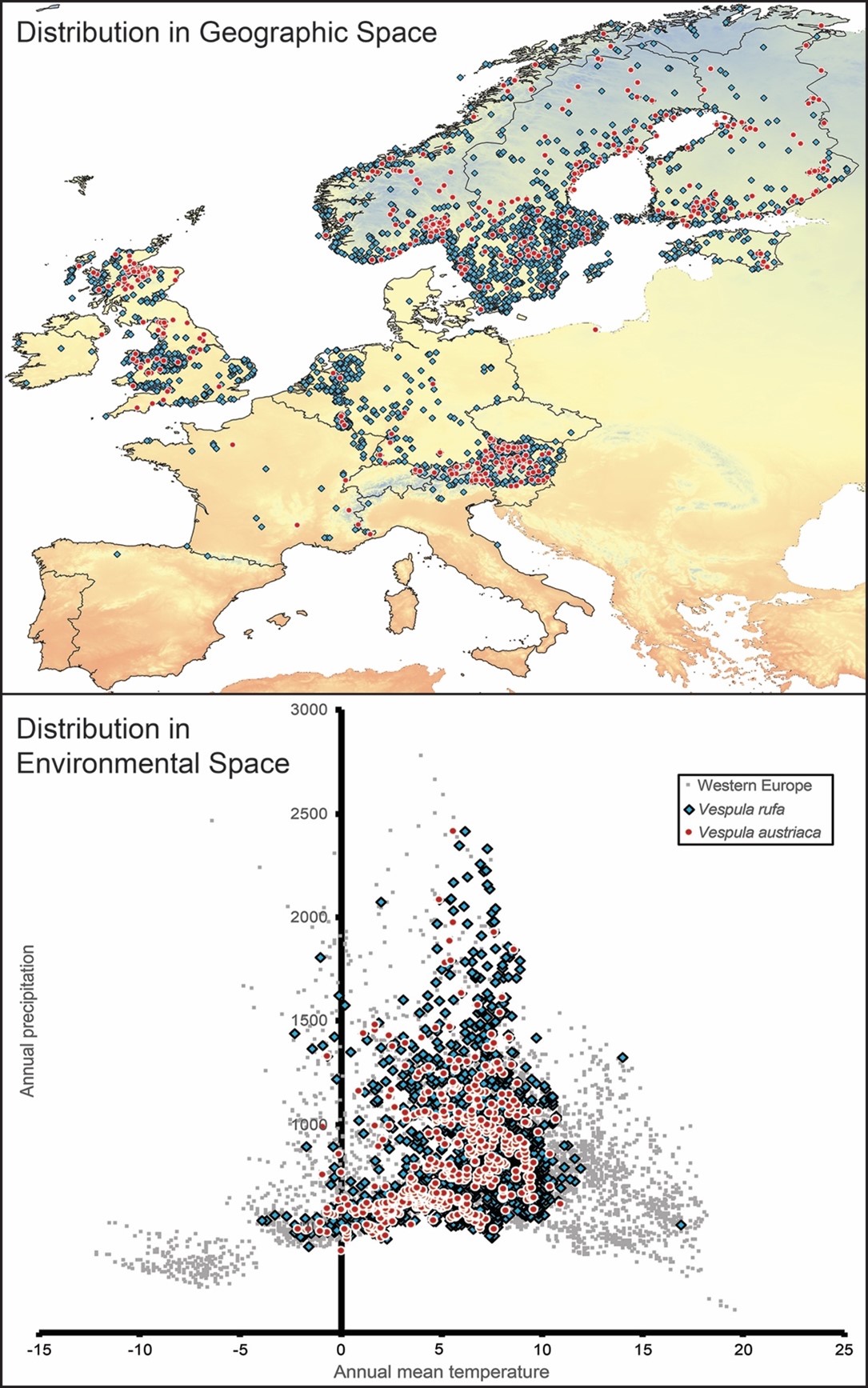
Figure 3. Summary of the distribution of one host-parasite system (Vespula rufa and V. austriaca, respectively, across western Europe, shown on top of the annual mean temperature data set (red = high, blue = low) (Hijmans et al., 2005). In the lower panel, the 2 species are shown in relation to the environments available across the region (in medium gray).
(Source: Adapted from Hijmans et al. (2005). License: CC BY-NC-SA 4.0.)
Published Examples
Parasitology has a rich history of interest in distributions and environmental constraints on distributions, yet it has not seen an abundance of distributional ecology studies, in the modern, quantitative sense. Where parasites have been analyzed in greatest detail is certainly as regards pathogenic organisms, including viruses (for example, Kearney et al., 2009; Oliveira et al., 2013; Campbell et al., 2015; Escobar et al., 2015a), bacteria (for example, Eisen et al., 2006; Giles et al., 2010; Escobar et al., 2015b), simple eukaryotes (for example, Foley et al., 2008; Kulkarni et al., 2010; Gurgel-Gonçalves et al., 2012; Escobar et al., 2014; Ramsey et al., 2015), and a cutting edge papers on macroparasites (for example, Botero-Cañola et al., 2019; Botero-Cañola and Gardner, 2023; Haverkost et al., 2010; Gentry et al., 2016). However, some of pathogen-related studies mentioned above generally assess the occurrence of the disease per se, and often neglect the independent distributional potentials of the parasite and host. That is, they treat the disease transmission system as a black box that results in human, other (non-human) animal, or plant disease (Peterson, 2014). Black box models have the advantage of integrating over the entire transmission cycle of a parasite or pathogen, but have the failing of not focusing on the ecological niche of any species in particular, and of being easily biased by regional differences in sampling intensity, diagnostic capacities, or reporting frequency (Waller et al., 2007).
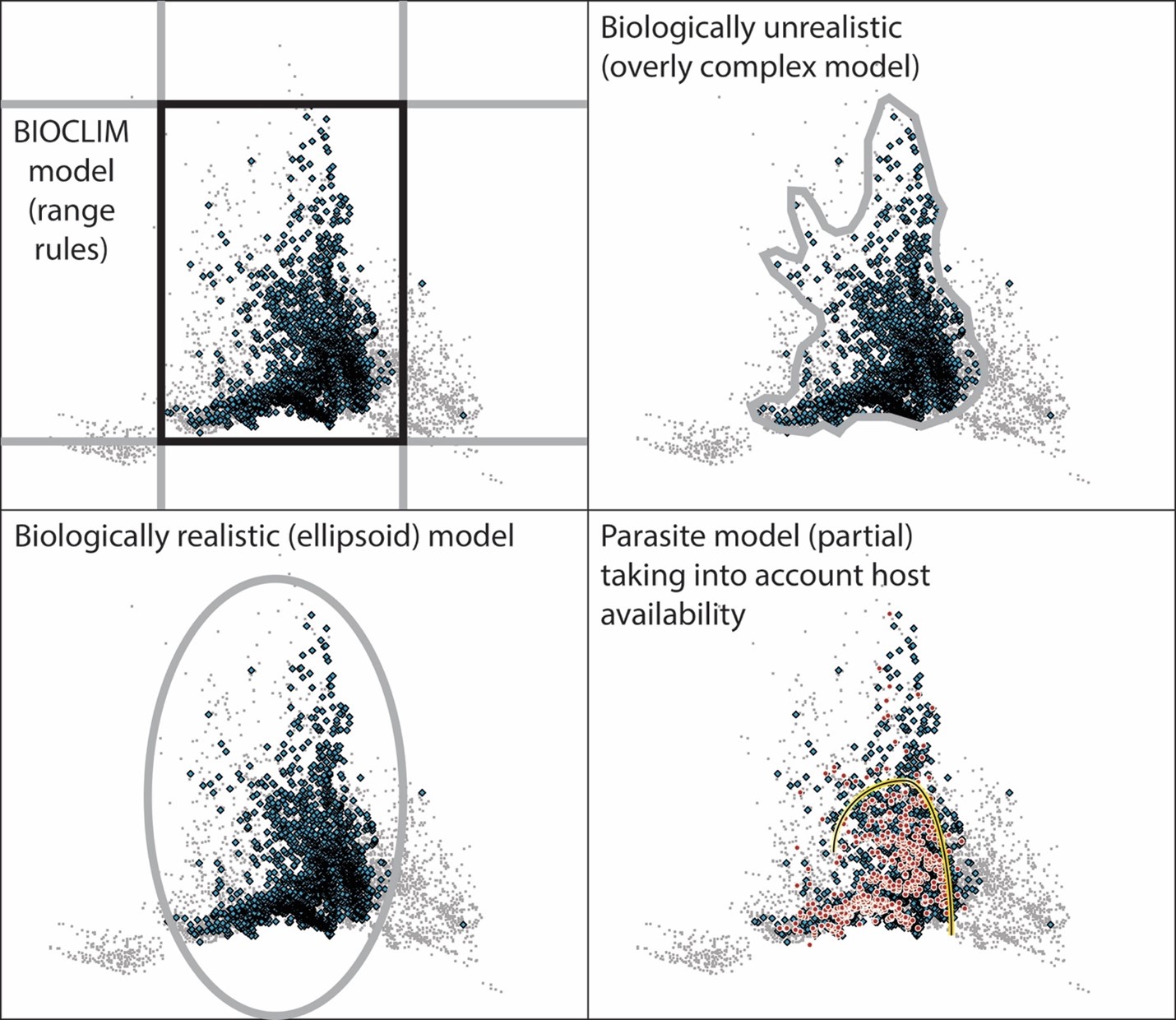
Figure 4. An illustration of methods and some key ideas in ecological niche modeling. Top panels and bottom-left panel are focused on Vespula rufa (the host species): Gray dots show the set of environments that is accessible to the species across western Europe, whereas the blue diamonds are the occurrences of the species. The gray and black lines show the set of environments that might be “chosen” as within the species niche under different approaches. Finally, the bottom-right panel shows the parasite (V. austriaca) distribution on top of that of the host and the available environments. The yellow-and-black line separates the distribution of the parasite (red points) from areas in which the host is available (blue diamonds), yet few parasite records are available (note that the great bulk of the parasite records comes from below the yellow-and-black curve), suggesting niche limits for the parasite, independent of the host’s niche.
(Source: A. T. Peterson, 2019. License: CC BY-NC-SA 4.0.)
Distributions
Most parasite-oriented studies in distributional ecology have focused on distributional questions. That is, most studies have taken known occurrences and have attempted to predict the full geographic distribution of the disease (for example, Sehgal et al., 2010; Machado-Machado, 2012). Rarer are studies that include careful testing with independent data (for example, Escobar et al., 2015a; Botero-Cañola et al., 2019; Botero-Cañola and Gardner, 2023). Other studies include model transfers to future conditions, where distributional shifts are anticipated that will likely manifest eventually as changing disease occurrence patterns (Rödder et al., 2010; Rose and Wall, 2011; Suwannatrai et al., 2017; Alkishe et al., 2018).
Perhaps most interesting is the potential for developing fine-resolution distributional summaries for species, even across complex and poorly sampled landscapes. Here, when fine-resolution occurrence data, such as those that are derived from GPS georeferencing for recent field records, are available, they can be integrated with equally fine-resolution environmental data deriving from remote sensing. The result is a highly precise and detailed mapping of the distributional potential of the species across broad landscapes, thanks to the pairing of fine-resolution data on both occurrence and environment. Examples include applications to understanding the spatial distribution of likely avian influenza risk across Southeast and East Asia (Gilbert et al., 2007; Xiao et al., 2007; Gilbert et al., 2008; Dhingra et al., 2016) and other regions (Bodbyl-Roels et al., 2011), fine-scale predictions of triatomine distributions in Mexico (López-Cárdenas et al., 2005), and others, although exploration of the full diversity of remote-sensing data products is likely still in its infancy in distributional ecological studies.
Finally, it is worth mentioning that studies of this general sort that are specifically interpreted in the context of infection risk—that is, including additional processing beyond just modeling the niche and estimating A in the BAM diagram—are relatively rare (Ostfeld et al., 2006; Estrada-Peña et al., 2014; Ostfeld et al., 2018). The ideas central to this step (that is, risk mapping) are treated in detail in a book-length contribution (Peterson, 2014).
Niches
On the niche and environment side, this suite of techniques has perhaps seen much less application to those questions. An early contribution (Costa et al., 2014) explored ecological niche variation within a key complex of vector insects that transmit Chagas disease, but failed to distinguish between fundamental and existing niches, which wasn’t well appreciated at that time. A later contribution, also focused on Chagas vectors, documented niche differentiation within the Triatoma dimidiata complex more rigorously (Gómez-Palacio et al., 2015), including detailed background similarity testing (Warren et al., 2008), to avoid misinterpreting existing niche differentiation as fundamental niche differentiation.
Niches and Distributions
On a more synthetic level, one suite of analyses has gone deep into the interaction between sampling and reporting of pathogen occurrences and their likely geographic distributions (Del Valle et al., 2018), with deep integration of dispersal opportunity and ecological niche, to get at transmission risk more or less rigorously (Escobar et al., 2016).
Another study, focused on the plague transmission system, assembled information on human cases, animal detections of the pathogen, and the broader distributions of the host mammal species, to test whether the distribution of plague is a function of the distributions of its hosts, or rather on its own distributional potential (Maher et al., 2010). This work was echoed later in an assessment of a plant-parasite system (Lira-Noriega and Peterson, 2014). Finally, one early analysis focused on using distributional estimates from ecological niche models to predict the mammal hosts of triatomine bugs in the Protracta group of species within the genus Triatoma, and the predictions turned out to be quite predictive of host-parasite associations (Peterson et al., 2002). This sort of deeper, and more synthetic, application of distributional ecology tools to parasite distributions is rare, but is quite promising as regards making concrete contributions to understanding parasite distributions.
For macroparasites, early explorations managed to outline the potential of these methods and demonstrate some of the interest in their potential (Haverkost et al., 2010), and regionally focussed studies have recently been published, focusing on a Echinococcus multilocularis, a pathogenic cestode by Botero-Cañola and colleagues (2019) and a general test of latitudinal variation in parasitism using museum collections based data (Botero-Cañola and Gardner, 2023). Meanwhile global geographic summaries of key groups have also been published (Feidas et al., 2014). Chaiyos and colleagues (2018) developed detailed niche models for a number of macroparasites in humans in Thailand and explored their results in both geographic and environmental spaces. Lira-Noriega and others (2013) developed detailed analyses to assess whether biotic drivers (that is, host associations) versus Grinnellian niches drove distributions of parasitic mistletoe distributions.
Future Perspectives
Distributional ecology has progressed from a descriptive effort (for example, making a map by hand) to a quantitative effort, and the quantitative approaches have moved from shots in the dark (“look, this works!”) to steps that are firmly based in ecological theory, in just a few decades. As such, the field is exciting and vibrant, and is seeing intensive research attention across many taxa and across many fields. Still, applications in parasitology have lagged somewhat, leaving many opportunities for exciting steps forward in understanding geographic and environmental distributions of many types of parasites.
Parasite applications in distributional ecology may be more complicated than most such studies, because of the frequent negation of the Eltonian Noise Hypotheses—that is, interactions with other species often do matter to parasites, at least in many cases. Indeed, one of the most useful testing frameworks has almost never been applied in parasitology: If one has a hypothesis about a biotic interaction, one can build ecological niche models that include and exclude that interacting species (for example, a host). One can then assess quantitatively whether the models with the interactor are better (for example, in predictive challenges, or in terms of maximum likelihood) than the models without the interactor (Atauchi et al., 2018). Such simple assessments have the potential eventually to understand some of the most fundamental elements of distributions of parasites—are their distributions governed by the niches of their hosts, or do they have meaningful niche constraints on their own?
More fundamentally, though, applications of ideas from distributional ecology to questions in parasitology must weigh very carefully the conceptual framework of the question, in order to proceed to deeper and more interesting questions. That is, a world of exciting questions abounds, such as the environmental dimensions of and constraints on the process of host-parasite co-speciation, or micro-scale versus macro-scale niche dimensions that may constrain parasite distributions at multiple scales, and how different types of niches (for example, realized or fundamental) may be broader or narrower at different spatial scales. The challenge, however, is to assemble a methodology that responds first to the conceptual foundations, and then is adapted and applied to the specific case of the parasite in question. Once such conceptual rigor is in hand, exciting distributional ecology results will emerge for parasitology.
Literature Cited
Alkishe, A. A., A. T. Peterson, and A. M. Samy. 2018. Climate change influences on the potential geographic distribution of the disease vector tick Ixodes ricinus. PLoS One 12: e0189092. doi: 10.1371/journal.pone.0189092
Anderson, R. P. 2017. When and how should biotic interactions be considered in models of species niches and distributions? Journal of Biogeography 44: 8–17. doi: 10.1111/jbi.12825
Atauchi, P. J., A. T. Peterson, and J. Flanagan. 2018. Species distribution models for Peruvian Plantcutter improve with consideration of biotic interactions. Journal of Avian Biology 49: jav-01617. doi: 10.1111/jav.01617
Barve, N., V. Barve, A. Jiménez-Valverde, A. Lira-Noriega, et al. 2011. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecological Modelling 222: 1,810–1,819. doi: 10.1016/j.ecolmodel.2011.02.011
Bodbyl-Roels, S., A. T. Peterson, and X. Xiao. 2011. Comparative analysis of remotely-sensed data products via ecological niche modeling of avian influenza case occurrences in Middle Eastern poultry. International Journal of Health Geographics 10: 21. doi: 10.1186/1476-072X-10-21
Botero-Cañola, S., A. T. Dursahinhan, S. E. Rácz, P. V. Lowe, et al. 2019. The ecological niche of Echinococcus multilocularis in North America: Understanding biotic and abiotic determinants of parasite distribution with new records in New Mexico and Maryland, United States. Therya 10: 91–102. doi: 10.12933/therya-19-749
Botero-Cañola, S., and S. L. Gardner. 2023. Tapping into natural history data to understand distribution of parasites. Parasitology 150: 723–733. doi: 10.1017/S0031182023000458
Campbell, L. P., C. Luther, D. Moo-Llanes, J. M. Ramsey, et al. 2015. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philosophical Transactions of the Royal Society B 370: 20140135. doi: 10.1098/rstb.2014.0135
Chaiyos, J., K. Suwannatrai, K. Thinkhamrop, K. Pratumchart, et al. 2018. MaxEnt modeling of soil-transmitted helminth infection distributions in Thailand. Parasitology Research 117: 3,507–3,517. doi: 10.1007/s00436-018-6048-7
Chapman, A. D. 2005. Principles and Methods of Data Cleaning, version 1.0. Global Biodiversity Information Facility, Copenhagen, Denmark.
Chapman, A. D., and J. Wieczorek, eds. 2006. Guide to best practices for georeferencing. Global Biodiversity Information Facility, Copenhagen, Denmark.
Cobos, M. E., L. Jiménez, C. Nuñez-Penichet, D. RomeroÁlvarez, et al. 2018. Sample data and training modules for cleaning biodiversity information. Biodiversity Informatics 13: 49–50. doi: 10.17161/bi.v13i0.7600
Colwell, R. K., and T. F. Rangel. 2009. Hutchinson’s duality: The once and future niche. Proceedings of the National Academy of Sciences of the United States of America 106: 19,644–19,650. doi: 10.1073/pnas.0901650106
Costa, J., L. L. Dornak, C. E. Almeida, and A. T. Peterson. 2014. Distributional potential of the Triatoma brasiliensis species complex at present and under scenarios of future climate conditions. Parasites and Vectors 7: 238. doi: 10.1186/1756-3305-7-238
Del Valle, S., B. H. McMahon, J. Asher, R. Hatchett, et al. 2018. Summary results of the 2014–2015 DARPA Chikungunya Challenge. BMC Infectious Diseases 18: 245. doi: 10.1186/s12879-018-3124-7
Dhingra, M. S., J. Artois, T. P. Robinson, C. Linard, et al. 2016. Global mapping of highly pathogenic avian influenza H5N1 and H5Nx clade 2.3. 4.4 viruses with spatial cross-validation. eLife 5: e19571.
Eisen, R. J., R. S. Lane, C. L. Fritz, and L. Eisen. 2006. Spatial patterns of Lyme disease risk in California based on disease incidence data and modeling of vector-tick exposure. American Journal of Tropical Medicine and Hygiene 75: 669–676. doi: 10.4269/ajtmh.2006.75.669
Elith, J., C. H. Graham, R. P. Anderson, M. Dudik, et al. 2006. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29: 129–151. doi: 10.1111/j.2006.0906-7590.04596.x
Escobar, L. E., and M. E. Craft. 2016. Advances and limitations of disease biogeography using ecological niche modeling. Frontiers in Microbiology 7: 1,174. doi: 10.3389/fmicb.2016.01174
Escobar, L. E., A. Lira-Noriega, G. Medina-Vogel, and A. T. Peterson. 2014. Potential for spread of White-nose Fungus (Pseudogymnoascus destructans) in the Americas: Using Maxent and NicheA to assure strict model transference. GeoHealth 9: 221–229. doi: 10.4081/gh.2014.19
Escobar, L. E., A. T. Peterson, M. Papeş, M. Favi, et al. 2015a. Ecological approaches in veterinary epidemiology: Mapping the risk of bat-borne rabies using vegetation indices and night-time light satellite imagery. Veterinary Research 46: 92. doi: 10.1186/s13567-015-0235-7
Escobar, L. E., H. Qiao, and A. T. Peterson. 2016. Forecasting Chikungunya spread in the Americas via data-driven, empirical approaches. Parasites and Vectors 9: 112. doi: 10.1186/s13071-016-1403-y
Escobar, L. E., S. J. Ryan, A. M. Stewart-Ibarra, J. L. Finkelstein, et al. 2015b. A global map of suitability for coastal Vibrio cholerae under current and future climate conditions. Acta Tropica 149: 202–211. doi: 10.1016/j.actatropica.2015.05.028
Estrada-Peña, A., R. S. Ostfeld, A. T. Peterson, R. Poulin, et al. 2014. Effects of environmental change on zoonotic disease risk: An ecological primer. Trends in Parasitology 30: 205–214. doi: 10.1016/j.pt.2014.02.003
Feidas, H., M. K. Kouam, V. Kantzoura, and G. Theodoropoulos. 2014. Global geographic distribution of Trichinella species and genotypes. Infection, Genetics and Evolution 26: 255–266. doi: 10.1016/j.meegid.2014.06.009
Fielding, A. H., and J. F. Bell. 1997. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environmental Conservation 24: 38–49.
Foley, D. H., T. A. Klein, H. C. Kim, R. C. Wilkerson, et al. 2008. Malaria risk assessment for the Republic of Korea based on models of mosquito distribution. US Army Medical Department Journal 6: PB8-08.
Franklin, J. 2010. Mapping Species Distributions: Spatial Inference and Prediction. Cambridge University Press, Cambridge, United Kingdom, 320 p.
Gentry, J., B. Sturm, and A. T. Peterson. 2016. Predictive mapping of transmission risk of a soil-transmitted helminth across East Africa from community survey data. Journal of Public Health in Developing Countries 2: 151–161.
Gilbert, M., X. Xiao, P. Chaitaweesub, W. Kalpravidh, et al. 2007. Avian influenza, domestic ducks and rice agriculture in Thailand. Agriculture, Ecosystems and Environment 119: 409–415.
Gilbert, M., X. Xiao, D. U. Pfeiffer, M. Epprecht, et al. 2008. Mapping H5N1 highly pathogenic avian influenza risk in Southeast Asia. Proceedings of the National Academy of Sciences USA 105: 4,769–4,774. doi: 10.1073/pnas.0710581105
Giles, J., A. T. Peterson, and A. Almeida. 2010. Ecology and geography of plague transmission areas in northeastern Brazil. PLoS Neglected Tropical Diseases 5: e925. doi: 10.1371/journal.pntd.0000925
Godsoe, W. 2010. I can’t define the niche but I know it when I see it: A formal link between statistical theory and the ecological niche. Oikos 119: 53–60. doi: 10.1111/j.1600-0706.2009.17630.x
Gómez-Palacio, A., S. Arboleda, E. Dumonteil, O. Triana, et al. 2015. Ecological niche and geographic distribution of the Chagas disease vector, Triatoma dimidiata (Reduviidae: Triatominae): Evidence for niche differentiation among cryptic species. Infection, Genetics and Evolution 36: 15–22. doi: 10.1016/j.meegid.2015.08.035
Grinnell, J. 1914. Barriers to distribution as regards birds and mammals. American Naturalist 48: 248–254. doi: 10.1086/279402
Grinnell, J. 1917a. Field tests of theories concerning distributional control. American Naturalist 51: 115–128. doi: 10.1086/279591
Grinnell, J. 1917b. The niche-relationships of the California Thrasher. Auk 34: 427–433.
Guisan, A., W. Thuiller, and N. E. Zimmermann. 2017. Habitat Suitability and Distribution Models: with Applications in R. Cambridge University Press, Cambridge, United Kingdom.
Gurgel-Gonçalves, R., C. Galvão, J. Costa, and A. T. Peterson. 2012. Geographic distribution of Chagas disease vectors in Brazil based on ecological niche modeling. Journal of Tropical Medicine 2012: 705326. doi: 10.1155/2012/705326
Haverkost, T. R., S. L. Gardner, and A. T. Peterson. 2010. Predicting the distribution of a parasite using the ecological niche model, GARP. Revista Mexicana de Biodiversidad 81: 895–902.
Hijmans, R., S. Cameron, J. Parra, P. Jones, et al. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1,965–1,978. doi: 10.1002/joc.1276
Hutchinson, G. E. 1957. Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology 22: 415–427.
Kearney, M., W. P. Porter, C. Williams, S. Ritchie, et al. 2009. Integrating biophysical models and evolutionary theory to predict climatic impacts on species’ ranges: The dengue mosquito Aedes aegypti in Australia. Functional Ecology 23: 528–538. doi: 10.1111/j.1365-2435.2008.01538.x
Kulkarni, M. A., R. E. Desrochers, and J. T. Kerr. 2010. High resolution niche models of malaria vectors in northern Tanzania: A new capacity to predict malaria risk? PLoS One 5: e9396. doi: 10.1371/journal.pone.0009396
Lira-Noriega, A., and A. T. Peterson. 2014. Range-wide ecological niche comparisons of parasite, hosts and dispersers in a vector-borne plant parasite system. Journal of Biogeography 41: 1,664–1,673. doi: 10.1111/jbi.12302
Lira-Noriega, A., J. Soberón, and C. P. Miller. 2013. Process-based and correlative modeling of desert mistletoe distribution: A multiscalar approach. Ecosphere 4: art99. doi: 10.1890/ES13-00155.1
López-Cárdenas, J., F. E. González-Bravo, P. M. Salazar-Schettino, J. C. Gallaga-Solórzano, et al. 2005. Fine-scale predictions of distributions of Chagas disease vectors in the state of Guanajuato, Mexico. Journal of Medical Entomology 42: 1,068–1,081. doi: 10.1093/jmedent/42.6.1068
Machado-Machado, E. A. 2012. Empirical mapping of suitability to dengue fever in Mexico using species distribution modeling. Applied Geography 33: 82–93. doi: 10.1016/j.apgeog.2011.06.011
Maguire, B. 1973. Niche response structure and the analytical potentials of its relationship to the habitat. American Naturalist 107: 213–246. doi: 10.1086/282827
Maher, S. P., C. Ellis, K. L. Gage, R. E. Enscore, et al. 2010. Range-wide determinants of plague distribution in North America. American Journal of Tropical Medicine and Hygiene 83: 736–742. doi: 10.4269/ajtmh.2010.10-0042
Mainali, K. P., D. L. Warren, K. Dhileepan, A. McConnachie, et al. 2015. Projecting future expansion of invasive species: Comparing and improving methodologies for species distribution modeling. Global Change Biology 21: 4,464–4,480. doi: 10.1111/gcb.13038
Muscarella, R., P. J. Galante, M. Soley-Guardia, R. A. Boria, et al. 2014. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods in Ecology and Evolution 5: 1,198–1,205. doi: 10.1111/2041-210X.12261
Nix, H. A. 1986. A biogeographic analysis of Australian elapid snakes. In R. Longmore, ed. Atlas of Elapid Snakes of Australia. Australian Government Publishing Service, Canberra, Australia, p. 4–15.
Oliveira, S. V., L. E. Escobar, A. T. Peterson, and R. Gurgel-Gonçalves. 2013. Potential geographic distribution of hantavirus reservoirs in Brazil. PLoS One 8: e85137. doi: 10.1371/journal.pone.0085137
Ostfeld, R. S., C. D. Canham, K. Oggenfuss, R. J. Winchcombe, et al. 2006. Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLoS Biology 4: e145.
Ostfeld, R. S., T. Levi, F. Keesing, K. Oggenfuss, et al. 2018. Tick-borne disease risk in a forest food web. Ecology 99: 1,562–1,573. doi: 10.1371/journal.pbio.0040145
Pavlovsky, E. N. 1966. Natural Nidality of Transmissible Diseases. University of Illinois Press, Urbana, Illinois, United States.
Peterson, A. T. 2008. Biogeography of diseases: A framework for analysis. Naturwissenschaften 95: 483–491. doi: 10.1007/s00114-008-0352-5
Peterson, A. T. 2011. Ecological niche conservatism: A time-structured review of evidence. Journal of Biogeography 38: 817–827. doi: 10.1111/j.1365-2699.2010.02456.x
Peterson, A. T. 2014. Mapping Disease Transmission Risk in Geographic and Ecological Contexts. Johns Hopkins University Press, Baltimore, Maryland, United States, 328 p.
Peterson, A. T. 2007. Why not WhyWhere: The need for more complex models of simpler environmental spaces. Ecological Modelling 203: 527–530. doi: 10.1016/j.ecolmodel.2006.12.023
Peterson, A. T., and A. G. Navarro-Sigüenza. 2009. Making biodiversity discovery more efficient: An exploratory test using Mexican birds. Zootaxa 2246: 58–66.
Peterson, A. T., A. G. Navarro-Sigüenza, and A. Gordillo-Martínez. 2016. The development of ornithology in Mexico and the importance of access to scientific information. Archives of Natural History 43: 294–304.
Peterson, A. T., V. Sánchez-Cordero, C. B. Beard, and J. M. Ramsey. 2002. Ecologic niche modeling and potential reservoirs for Chagas disease, Mexico. Emerging Infectious Diseases 8: 662–667. doi: 10.3201/eid0807.010454
Peterson, A. T., J. Soberón, R. G. Pearson, R. P. Anderson, et al. 2011. Ecological Niches and Geographic Distributions. Princeton University Press, Princeton, New Jersey, United States.
Peterson, A. T., J. Soberón, and V. Sánchez-Cordero. 1999. Conservatism of ecological niches in evolutionary time. Science 285: 1,265–1,267. doi: 10.1126/science.285.5431.1265
Pulliam, H. R. 2000. On the relationship between niche and distribution. Ecology Letters 3: 349–361. doi: 10.1046/j.1461-0248.2000.00143.x
Qiao, H., J. Soberón, and A. T. Peterson. 2015. No silver bullets in correlative ecological niche modeling: Insights from testing among many potential algorithms for niche estimation. Methods in Ecology and Evolution 6: 1,126–1,136. doi: 10.1111/2041-210X.12397
Ramírez-Gil, J. G., J. G. Morales, and A. T. Peterson. 2019. Current and potential distributions of the eight most important diseases in Hass [sic; Haas] avocado in Antioquia, Colombia. Journal of Plant Protection Research 59: 214–228. doi: 10.24425/jppr.2019.129288
Ramsey, J. M., A. T. Peterson, O. Carmona-Castro, D. A. Moo-Llanes, et al. 2015. Atlas of Mexican Triatominae (Reduviidae: Hemiptera) and vector transmission of Chagas disease. Memorias del Instituto Oswaldo Cruz 110: 339–352. doi: 10.1590/0074-02760140404
Raxworthy, C. J., E. Martínez-Meyer, N. Horning, R. A. Nussbaum, et al. 2003. Predicting distributions of known and unknown reptile species in Madagascar. Nature 426: 837–841. doi: 10.1038/nature02205
Reddy, S., and Á. S. Nyári. 2015. Novel insights into the historical biogeography of the Streak-breasted Scimitar-babbler complex (Aves: Timaliidae: Pomatorhinus ruficollis complex). Current Zoology 61: 910–921. doi: 10.1093/czoolo/61.5.793
Rödder, D., J. Kielgast, and S. Lötters. 2010. Future potential distribution of the emerging amphibian chytrid fungus under anthropogenic climate change. Diseases of aquatic organisms 92: 201–207. doi: 10.3354/dao02197
Rose, H., and R. Wall. 2011. Modelling the impact of climate change on spatial patterns of disease risk: Sheep blowfly strike by Lucilia sericata in Great Britain. International Journal of Parasitology 41: 739–746. doi: 10.1016/j.ijpara.2011.01.012
Samy, A. M., S. M. Thomas, A. A. E. Wahed, K. P. Cohoon, et al. 2016. Mapping the global geographic potential of Zika virus spread. Memórias do Instituto Oswaldo Cruz 111: 559–560. doi: 10.1590/0074-02760160149
Sánchez-Tapia, A., M. F. de Siqueira, R. O. Lima, F. S. M. Barros, et al. 2017. Model-R: A framework for scalable and reproducible ecological niche modeling. In Latin American High Performance Computing Conference, p. 218–232. Springer, Cham, Switzerland.
Saupe, E. E., V. Barve, C. E. Myers, J. Soberón, et al. 2012. Variation in niche and distribution model performance: The need for a priori assessment of key causal factors. Ecological Modelling 237: 11–22. doi: 10.1016/j.ecolmodel.2012.04.001
Sehgal, R. N. M., W. Buermann, R. J. Harrigan, C. Bonneaud, et al. 2010. Spatially explicit predictions of blood parasites in a widely distributed African rainforest bird. Proceedings of the Royal Society B: Biological Sciences 278: 1,025–1,033. doi: 10.1098/rspb.2010.1720
Sillero, N. 2011. What does ecological modelling model? A proposed classification of ecological niche models based on their underlying methods. Ecological Modelling 222: 1,343–1,346. doi: 10.1016/j.ecolmodel.2011.01.018
Soberón, J., and M. Nakamura. 2009. Niches and distributional areas: Concepts, methods, and assumptions. Proceedings of the National Academy of Sciences USA 106: 19,644–19,650.doi: 10.17161/bi.v2i0.4
Soberón, J., and A. T. Peterson. 2005. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodiversity Informatics 2: 1–10.
Suwannatrai, A., K. Pratumchart, K. Suwannatrai, K. Thinkhamrop, et al. 2017. Modeling impacts of climate change on the potential distribution of the carcinogenic liver fluke, Opisthorchis viverrini, in Thailand. Parasitology research 116: 243–250. doi: 10.1007/s00436-016-5285-x
Taylor, L. H. 1939. Observations on social parasitism in the genus Vespula Thomson. Annals of the Entomological Society of America 32: 304–315.
Tingley, M. W., W. B. Monahan, S. R. Beissinger, and C. Moritz. 2009. Birds track their Grinnellian niche through a century of climate change. Proceedings of the National Academy of Sciences USA 106: 19,637–19,643. doi: 10.1073/pnas.0901562106
Waller, L., B. Goodwin, M. Wilson, R. Ostfeld, et al. 2007. Spatio-temporal patterns in county-level incidence and reporting of Lyme disease in the northeastern United States, 1990–2000. Environmental and Ecological Statistics 14: 83–100. doi: 10.1007/s10651-006-0002-z
Warren, D. L., R. E. Glor, and M. Turelli. 2008. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 62: 2,868–2,883. doi: 10.1111/j.1558-5646.2008.00482.x
Xiao, X., M. Gilbert, J. Slingenbergh, F. Lei, et al. 2007. Remote sensing, ecological variables and wild bird migration related to outbreaks of highly pathogenic H5N1 bird flu. Journal of Wildlife Diseases 43 (Supplement): S40–S46.
Adjective
From Greek: a = without; bios = life
Definition: Pertaining to, or characterized by, the absence of life
Adjective
From Greek: bios = life
Definition: Of or pertaining to life
Noun
From Latin: trans = across; mittere = to send
Definition 1: Horizontal: the transfer of an infectious agent from one organism to another
Definition 2: Vertical: transmission from one generation to another

