9 The Coccidia Proper: Important Apicomplexa Other than Haemoprotozoa
Donald W. Duszynski
Phylum Myzozoa
Subphylum Apicomplexa
History of the Term Apicomplexa
Taxonomy addresses the principles of scientific classification by discovering, observing, defining characters, ordering into groups, naming individual organisms that are clearly different within those groups, and archiving type specimens as appropriate in accredited museums. Historically, all living things defined as animals (that is, non-plants), were placed in 1 of 2 groups: Protozoa (meaning single-celled protists) or metazoa (meaning multicellular animals). Omitting hierarchical names (kingdom, phylum, class, and so on) for the moment, all protozoa were ordered into 1 of 4 groups based on how they moved, or didn’t: Ciliates (which have cilia), amoeba (which have pseudopodia), flagellates (which have flagella), and a catch-all category called the Sporozoa, most of which (but not all) had spores and some of which (myxosporidia and microsporidia) were not even remotely related to the spore-formers.
As knowledge increased, the name Sporozoa became unwieldy because it did not suggest or represent true evolutionary relationships between the organisms included therein. The widespread use of Transmission Electron Microscopy (TEM) for biological specimens began in the 1950s and continued throughout the 1960s and 1970s; many of these studies examined the fine structure of zoites belonging to a plethora of different protozoans. Eventually, a pattern began to emerge that revealed several common, consistently-shared structures (for example, polar rings, rhoptries, micronemes, and often a conoid) at the more pointed end (now termed anterior) of certain life stages (Figure 1). When present, these structures, in whatever combination, were termed the apical complex. At that time, protozoologists working on parasites sought a more phylogenetically relevant suite of characters to define their organisms, and Norman D. Levine, from the University of Illinois, came up with the name Apicomplexa to unify them. This complex structure is now known to be the focus of events during host cell penetration and establishment of the parasite within the cells of the host.
Introduction to the Apicomplexa
The protozoan group Apicomplexa Adl et al., 2005 (Levine, 1980) contains many obligate intracellular parasites including such diverse organisms as Coccidia, gregarines, haemosporoids, piroplasms, and cryptosporids, all united not by their biology or life histories, but by the presence of their unique apical complex. This complex collection of protozoans is subdivided into 2 major assemblages based on the presence or absence of a conoid in their apical complex. The Aconoidasida Mehlhorn et al. 1980 [= Hematozoa Vivier, 1982] all lack a conoid in their asexual motile stages, and include the Haemosporida Danilewsky, 1885 (for example, Plasmodium, Haemoproteus, Leucocytozoon, and others) and Piroplasmorida Wenyon, 1926 (for example, Babesia, Theileria, and others).
Members of the second major grouping, Conoidasida Levine, 1988, all have a complete apical complex that includes a hollow, truncated conoid in all or most of their asexual motile stages, along with other unifying features. This paraphyletic lineage includes 3 groups: Gregarinasina Dufour, 1828; the monogeneric family Cryptosporididae Tyzzer, 1907; and Coccidia Leuckart, 1879 according to Adl and colleagues (2012). Of the 2 Conoidasida groups that will not be covered in detail here, the gregarines parasitize invertebrates, and Cryptosporidium species, which were once considered to be atypical Coccidia, are most closely related to the gregarines and not the Coccidia (Cavalier-Smith, 2014; Thompson et al., 2016).
Before delving into the Coccidia, the history of taxonomic placement of the former Cryptosporididae will be discussed briefly. Bull and others (1998) first noticed there was serological cross-reactivity between anti-Cryptosporidium monoclonal antibody and sporocysts of the gregarine Monocystis, an observation mostly overlooked—or ignored—at the time. The next year, when sequencing SSU rDNA, Carreno and others (1999) inferred that Cryptosporidium was more closely related to gregarines than to Coccidia by phylogenetic analysis of apicomplexan parasites. Based on this and other molecular congruences, and on biological and behavioral similarities, Cavalier-Smith (2014) established a new subclass, Orthogregarinia, for Cryptosporidium and its most closely related gregarines, which include epicellular parasites of vertebrates possessing a gregarine-like feeder organelle and lacking an apicoplast (which is a relict, non-photosynthetic plastid found in most apicomplexan parasites). In addition to the SSU-rDNA sequencing evidence, Cryptosporidium shares biological features with gregarines including its epicellular location, connection to the host cell via a myzocytosis-like feeding mechanism, heterogeneity of trophozoite cell shape, and other structural similarities (see Thompson et al., 2016). Gliding movements seen in different trophic stages of Cryptosporidium species are behavioral features that also are similar to gliding movements exhibited by some gregarines (Borowski et al., 2008; 2010; Valigurová et al., 2013).
The Coccidia
Coccidia are united by having mature gametes that develop intracellularly, microgametocytes that usually produce many microgametes, and non-motile zygotes that mostly contain sporocysts within their oocysts. There are 2 Coccidia lineages: Adeleorina Léger, 1911 and Eimeriorina Léger, 1911. The Adeleorina has about 7 families, 2 of which each contain a genus of important parasites of vertebrates, Hepatozoidae Wenyon, 1926 (genus Hepatozoon) and Klossiellidae Smith and Johnson, 1902 (genus Klossiella). The Eimeriorina has 10–12 recognized families, 2 with multiple genera containing important parasites of vertebrates. The Eimeriidae has about 20 genera, but only 6 will be mentioned, to illustrate their diversity, namely, Acroeimeria, Caryospora, Choleoeimeria, Cyclospora, Eimeria, and Isospora. The Sarcocystidae has 7 genera of which 5 have extremely important parasites of humans and/or their domestic animals, namely, Besnoitia, Cystoisospora, Neospora, Sarcocystis, and Toxoplasma.
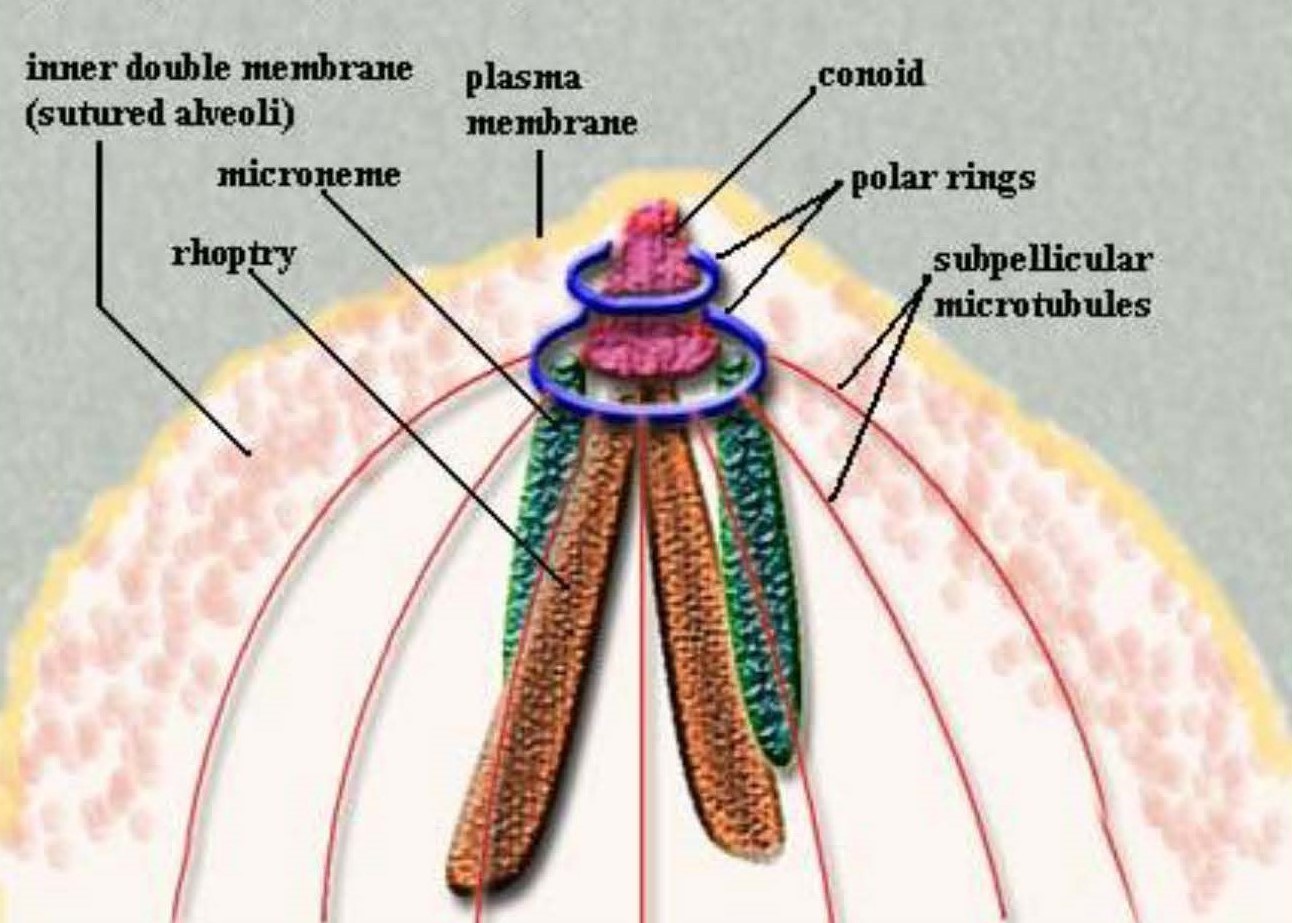
Figure 1. Apical complex structures at the anterior end of a coccidian zoite.
(Image source: Clowes et al., 2006. License: CC BY.)
Important Genera, Relation to Other Species, and Basic Life Histories
The apicomplexan genera with species that are important parasites of humans and/or their domestic, companion, and wild animals are highlighted in this section in the same taxonomic sequence outlined above:
Conoidasida Levine, 1988
Coccidia Leuckart, 1879
Adeleorina Léger, 1911
The Adeleorina is a poorly understood group of apicomplexan parasites. Members are united biologically by use of syzygy, a characteristic method of gamete formation by which both macro- and microgamonts are pressed together during their development (Adl et al., 2012). The Adeleorina has 7 families of Coccidia and includes those with both homoxenous and heteroxenous life cycles (Barta, 2000). In heteroxenous species, the conjugation of gamonts and subsequent sporogony most often occur within an invertebrate definitive host and (mechanical) vector; the oocysts formed contain numerous sporocysts, and sporozoites are found in the hemocoel of the definitive host (Craig, 2001). Once the vector is ingested, sporozoites are released, after which they penetrate the gut of the vertebrate intermediate host and enter the bloodstream to reach leukocytes and cells throughout the body where they undergo merogony. Many of the species in this group have morphologically distinct meronts and merozoites during their asexual reproduction, which occurs in the vertebrate (that is, intermediate) host. The first-generation meronts (M1) produce large merozoites (m1) that are thought to initiate a second round of merogony in which the M2 produce smaller m2s, which then become the progenitors of gamonts (Barta, 2000). Merogony in the tissues ultimately gives rise to gamonts in white blood cells (WBC) and tissue cysts; these tissue cysts may be a stage that can be transmitted by predation, but this remains to be determined (Craig, 1990; 2001).
Family Hepatozoidae Wenyon, 1926
This family has a single genus, Hepatozoon Wenyon, 1926b, with more than 300 described species (Baneth et al., 2007; Ivanov and Tsachev, 2008). Species in this genus infect various vertebrates including amphibians, reptiles, birds, and mammals, which are their intermediate hosts. The definitive hosts for these species are invertebrates that include mites, ticks, and various insects, and infection of the vertebrate host occurs when it ingests the infected invertebrate (not by its bite). Barta (2000) suggested the genus is paraphyletic. Hepatozoon canis is one important species in this genus since it can parasitize a favorite companion animal, the domestic dog.
Genus Hepatozoon Wenyon, 1926
Hepatozoon canis (James, 1905) Wenyon, 1926, can cause serious, life-threatening illness in vertebrates. In addition to dogs, it has been found parasitizing cheetahs, coyotes, jackals, foxes, hyenas, lions, and leopards (each as intermediate hosts) and has a worldwide distribution wherever its definitive host, the brown dog tick Rhipicephalus sanguineus (Latreille, 1806) is found. Note that other tick species also can serve as definitive hosts. Its prevalence in infected canid populations often is modest but also may be quite high. For example, Conceição-Silva and colleagues (1988) found 143 of 301 (48%) red foxes in Portugal to be infected while only 50 of 1,752 (3%) domestic dogs from the same area were infected. O’Dwyer and colleagues (2001) examined blood smears of dogs from rural areas of 7 counties in Rio de Janeiro state, Brazil, and identified H. canis in 98 of 250 (12%) dogs. Cardoso and others (2014) detected H. canis in 68 of 90 (76%) red foxes from 8 districts in Portugal, using both molecular (PCR amplification of 18S rRNA gene fragments) and histopathological sections of multiple tissues (bone marrow, heart, hind leg muscle, jejunum, kidney, liver, lung, popliteal or axillary lymph nodes, spleen, and/or tongue). Furtado and others (2017) collected blood samples from domestic dogs from 3 regions of Brazil; 81 of 129 (63%) dogs were positive for H. canis, as determined by PCR nucleotide sequences of the 18S rRNA gene of Hepatozoon.
In the life cycle of Hepatozoon canis in vertebrates (Figure 2), monozoic cysts have been found in the spleen, meronts and merozoites in the spleen, lymph nodes, lungs, liver, bone marrow, and gamonts and/or gametocytes in the cytoplasm of neutrophils and monocytes. Once ingested by the tick definitive host, gamonts need about 24 hours to free themselves from vertebrate white blood cells and soon thereafter they align side-by-side in syzygy. At 48 hours in the tick, 2 types of cells are present: 1. Elongated cells with an eccentric nucleus, presumed to be microgametes, and 2. more rounded cells, also with an eccentric nucleus, presumed to be macrogametes. At 4 days, zygotes (early oocysts) are formed; by 5 days, oocyst wall and sporocyst formation have begun (Baneth et al., 2007) and these stages are extracellular, not within tick host cells. Vincent-Johnson and others (1997) measured H. canis oocysts and reported that they were mostly spheroidal, 215 × 193 (160–325 × 138–258) μm with sporocysts that were 36 × 26 (29–41 × 17–30) μm.
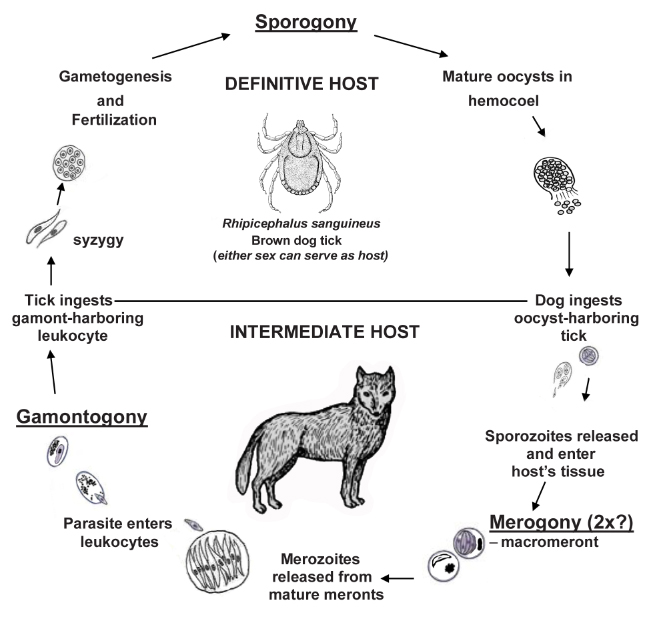
Figure 2. Diagrammatic drawing of the life cycle of Hepatozoon canis in dogs.
(Image sources: Tick, Pratt and Littig, 1962. Dog, V. Rausch, 1952. Other Figures, originals by S. L. Gardner, 2023. Tick image public domain; all other images, CC BY-NC-SA 4.0.)
Infection with Hepatozoon canis in dogs (and other vertebrates) ranges from being asymptomatic with low-level parasitemia, to a severe, life-threatening illness with fever, lethargy, anemia, and emaciation with very high parasitemia (Baneth et al., 2007). Sakuma and colleagues (2011) listed the characteristic hematological abnormalities in H. canis infections to include nonregenerative anemia, thrombocytopenia, neutrophilia, hyperproteinemia, hypoalbuminemia, polyclonal gammopathy, and increased concentrations of serum creatine kinase and alkaline phosphatase.
Hepatozoon species — Learn More
Interested readers can find more detailed information on this and other Hepatozoon species in dogs, cats, and other carnivores in Duszynski et al., 2018. If interested in various tissue stages, a picture of a meront in the spleen of a dog from the Philippines is shown in Vincent-Johnson et al., 1997, Figure 5; developmental stages in the tick and scanning electron microscopic images of oocysts and sporocysts in ticks are found in Baneth et al., 2007, Figures 2–13 and Figures 14–17, respectively.
Family Klossiellidae Smith and Johnson, 1902
This family also has a single genus, Klossiella Smith and Johnson, 1902, and it contains about 18 named species that infect primarily mammals, in which it invariably undergoes asexual and sexual development in the kidneys. For example, K. muris is found in the kidneys of house and lab mice (Mus musculus), K. cobayae in the capillaries of the guinea pig (Cavia porcellus) kidney, and K. equi in the kidney of asses (Equus asinus) and horses (Equus caballus) (Levine, 1973; Levine and Ivens, 1965). Levine and Ivens (1965) reviewed the highlights of Smith and Johnson’s (1902) discovery and the known developmental stages of this unusual coccidian. One example of a Klossiella species that infects the common opossum will suffice to illustrate this very interesting parasite family.
Genus Klossiella Smith and Johnson, 1902
Scorza and colleagues (1957) described Klossiella tejerai from a single common opossum Didelphis marsupialis (Linneas, 1758) in Venezuela. To date, K. tejerai only has been found in 2 other instances: In 4 of 10 (40%) of D. marsupialis in Panama (Edgcomb et al., 1976) and in 1 of 20 (5%) in big-eared opossums, Didelphis aurita, from Brazil (Spitz dos Santos, 2014). It is surmised that both asexual (merogony) and sexual (gamogony) stages are found within epithelial cells of the kidneys and associated ducts and tubules. The life cycle is direct (Figure 3) with very large oocysts, 72 × 47 (57–103 × 36–57) μm, that are irregular in shape, sporulation (sporogony) is endogenous producing 12–30 sporocysts, 20.4 × 12.7 (19–22 × 12–14) μm, each with 8–20 sporozoites.
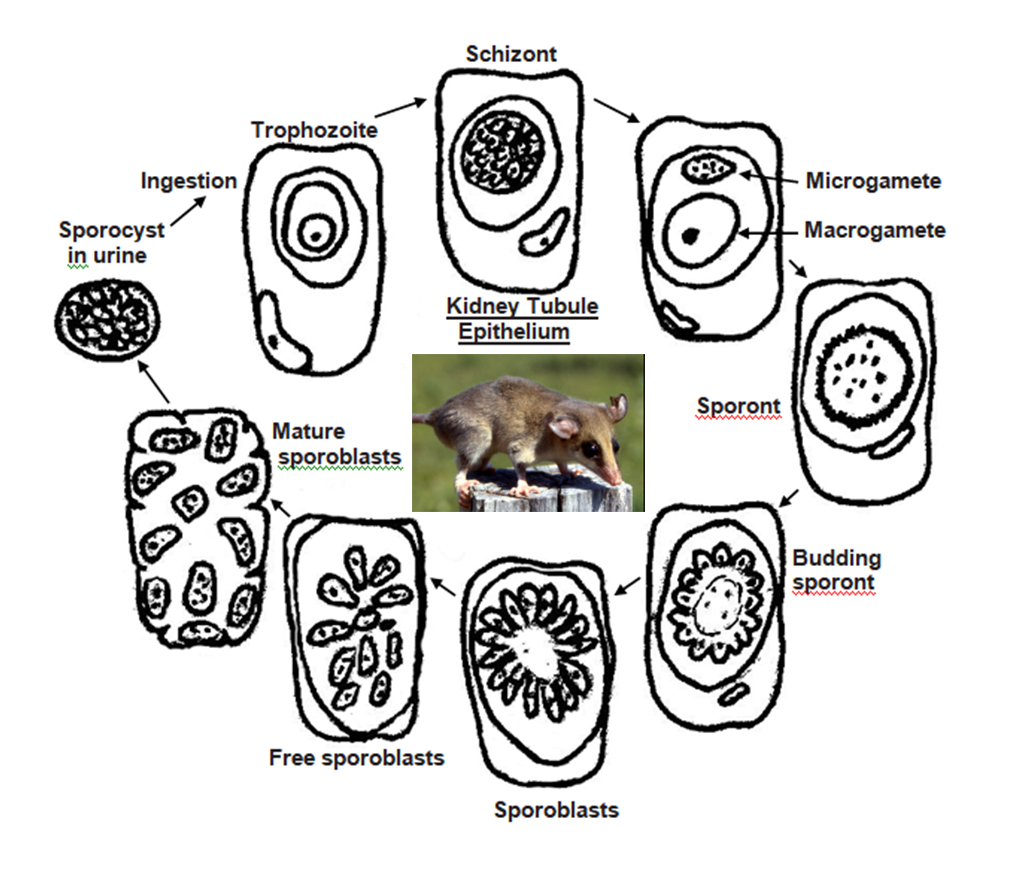
Figure 3. Diagrammatic drawing of the life cycle of Klossiella tejerai in opossums, Didelphis sp.
(Drawings by Duszynski. Photo by S. L. Gardner, 1993. License for all: CC BY-NC-SA 4.0.)
A Cautionary Example — Learn More
Edgcomb and others (1976) were among the first investigators to talk about pathological changes due to Klossiella species. They said (Edgcomb et al., 1976, p. 316–317), “Passage of schizonts (= meronts) and merozoites through the glomerular membranes occurs without inflammation and hemorrhage. These forms of the parasites evidently have membranes that permit their passage through the entire glomerular wall with restoration of the wall to an intact functional state after passage.” This seems an odd interpretation from observing just a few tissue sections. It can be envisioned how merozoites can penetrate cell membranes, but not meronts. They went on to say (Edgcomb et al., 1976, p. 317), “The invasion of tubular epithelial cells by gametes, particularly by macrogametes, is associated with ballooning necrosis of the invaded cells.” Spitz dos Santos and colleagues (2014) cautioned that Edgcomb and colleagues (1976) may have misinterpreted their photomicrographs.
Klossiella Species — Study It
Clearly, studying Klossiella species in the kidneys of vertebrates is an area ripe with potential rewards for new information. Parasitologists should begin to incorporate collecting urine into their field protocols to gain a sense of what oocysts and sporocysts of Klossiella really look like, and what variation can exist among species. Collecting kidney and related tissue samples for squash preparations/smears to be stained, and blocks of kidney to be fixed, embedded, sectioned, and prepared for histological examination (light microscopy (LM), transmission electron microscopy (TEM), or scanning electron microscopy (SEM)) will be critical. It will be an innovative milestone when someone finally infects several specimens of a vertebrate species with Klossiella oocysts/sporocysts, and then traces the sequential development over time of a complete life cycle within their kidneys. And, of course, it is imperative that DNA be collected and sequenced to gain an exact sense of the nature and affinity of these very interesting parasites—about which so little is known—to other species groups of the Apicomplexa. There are certainly a vast number of potential and obvious research projects available within this system to explore and problems to solve. This presents a wonderful opportunity, especially for graduate students who are teaching, to recruit undergraduates to help them with both field and lab work.
Eimeriorina Léger, 1911
Eimeriidae Minchin, 1903
The Eimeriorina contains species that all undergo merogony (asexual), gamogony (sexual), and sporogony (spore formation) during their life cycle. Members of the Eimeriidae all are homoxenous (direct life cycle), with merogony, gamogony, and the formation of oocysts occurring within the same host. Oocysts then leave the host, via the feces, and usually are unsporulated (= undeveloped, non-infective), but with a few exceptions (Choleoeimeria). The development of a genetically determined number of sporocysts and sporozoites within each oocyst occurs outside the host if/when environmental conditions (oxygen, moisture, and temperature) are appropriate (see Figure 4).
Genera Acroeimeria Paperna and Landsberg, 1989 and Choleoeimeria Paperna and Landsberg, 1989 (Figure 4)
The sporulated oocysts of Acroeimeria and Choleoeimeria are similar to those of Eimeria (see below) in that they all possess 4 sporocysts, each with 2 sporozoites, but all of their sporocysts lack a Stieda body, while most eimerians produce sporocysts with a Stieda body. Bovee and Telford (1965, p. 93) were the first to see a possible relationship between the shape of lizard Eimeria spp. oocysts and their site of infection when they wrote,
Eimeria spp. of lizards which form nearly spherical or elliptical oocysts with index 1.4 or less are inhabitants of the small intestine (if site of infection is known). Those of greater index, that is, long-ellipsoid or cylindrical form, are parasites of the biliary tract and particularly the gall bladder. The significance of this size-shape relationship to site is unknown.
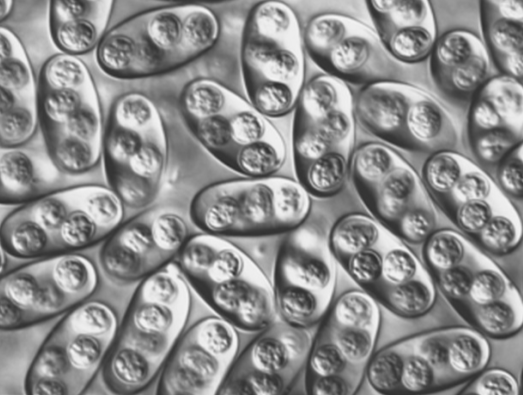
Figure 4. Elongate-ellipsoidal sporulated oocysts of a Choleoeimeria species in the bile duct and gallbladder from a colubrid snake Masticophis flagellum from Texas.
(Original photomicrograph, Duszynski and Upton, 2010. License: CC BY-NC-SA 4.0.)
Three decades later, Paperna and Landsberg (1989) reexamined the relationship between location of endogenous development of Eimeria species in geckos, their shape, and sporocyst structures. To accommodate their observations, they erected 2 new genera, Acroeimeria and Choleoeimeria. Both of their new genera had the general characteristic of the Eimeriidae (above). However, they defined Acroeimeria to have round or ovoidal oocysts with a length/width (L/W) ratio < 1.8, and all of their endogenous development (meronts, gamonts) “at the microvillous zone of the host cell and enclosed in the host cell microvillous boundary, causing the host cell to extend above the intestinal mucosal surface,” and sporulation was exogenous. Choleoeimeria was defined to have cylindroid to ovoidal oocysts with a L/W ratio always > 1.4 (up to 2.2), endogenous development (meronts, gamonts) in the gallbladder (as far as was known then), development that induced hypertrophy and displacement of epithelial host cells above their original cellular layer, and sporulation was endogenous in the gallbladder and gut lumen. Thus, Acroeimeria sporulated oocysts looked like Eimeria oocysts, but their endogenous developmental processes were unique.
Localization of endogenous development in the microvillous zone of intestinal epithelial cells was described earlier in fish eimerians by Dyková and Lom (1981) who proposed a new genus, Epieimeria, to accommodate these presumably epicytoplasmic piscine eimerians. However, Benajiba and others (1994) noticed that Epieimeria showed both epicytoplasmic and intracytoplasmic endogenous development, and Paperna (1991), using TEM, showed both epicytoplasmic and intracytoplasmic endogenous stages that develop within a parasitophorous vacuole, which makes them only intracytoplasmic. Thus, epicytoplasmic endogenous development did not occur in fish Epieimeria and this urged Benajiba and others (1994) to suppress the genus name and reassign all Epieimeria species back to the genus Eimeria. Several years later, Lainson and Paperna (1999), for unexplained reasons, changed their original definition of Acroeimeria slightly by stating that it, “Develops immediately beneath the brush-border of the intestinal epithelial cell” (p. 151), and this begs the question, whether or not Acroeimeria, as defined to have only epicytoplasmic endogenous stages (Paperna and Landsberg, 1989), also should be suppressed. We now know that some species of Acroeimeria exhibit both epicytoplasmic (typical of Acroeimeria) and intracytoplasmic (typical of Eimeria) endogenous development (unpublished data), a situation very similar to the piscine Epieimeria story. No one yet has done a careful molecular characterization of Acroeimeria versus other coccidians in saurian species.
The Choleoeimeria story is much simpler. Development of these species in the gallbladder and associated ducts, with the production of elongate-ellipsoidal or cylindroidal oocysts (Figure 4) that mostly sporulate endogenously seem to be conditions accepted by most of those who study lizard parasites, so far. Assigning species to Acroeimeria is more difficult and requires studying not only oocyst morphology, but structural information must be supported by information on the location and stages of endogenous development, and (multiple) gene sequencing whenever possible. As a practical matter, unless information on endogenous development and/or partial gene sequence can be obtained to support morphology of saurian coccidia recovered from the feces, the species names used should almost automatically be placed in the Eimeria.
Genus Caryospora Léger, 1904
The Caryospora genus is a really intriguing group of apicomplexan parasites. Caryospora species are mostly parasites of reptiles (predominantly snakes, but also lizards, turtles) and birds, and 1 species has been reported from mammals. Species assigned to this genus have 2 unusual features. First, their sporulated oocysts (Figures 5A–B) have only 1 sporocyst, which contains 8 sporozoites. Interestingly, those species described from reptiles almost always have a prominent Stieda body/substieda body complex while most of those caryosporans described from birds do not have a Stieda body/substieda body at the more pointed end. Although some species in this genus utilize life cycles similar to Eimeria and Isospora species, the second unique feature is that several Caryospora species from snakes are facultatively heteroxenous. In this type of life cycle, both an enteric phase in a snake host and a non-intestinal phase in rodents have been described (Figure 5C).
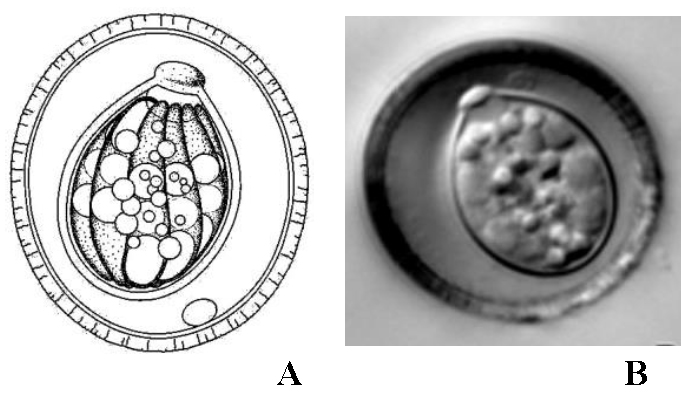
Figures 5A–B. A) Line drawing of the sporulated oocyst of Caryospora duszynskii. B) Photomicrograph of a sporulated oocyst of C. duszynskii.
(Both Figures from a colubrid snake, in Duszynski and Upton, 2010. License: CC BY-NC-SA 4.0.)
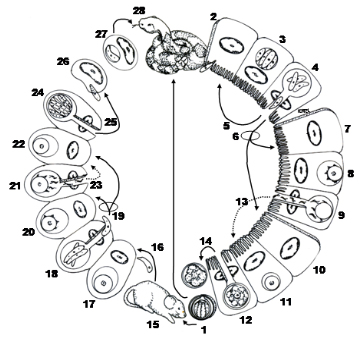
Figure 5C. Life cycle of a Caryospora species with both direct and facultatively heteroxenous life cycle components. 1) Typical sporulated oocyst which may be ingested by the snake definitive host in which case the parasite will undergo an enteric life cycle similar to Eimeria and Isospora species. 2) Sporozoites excyst in the intestine, penetrate epithelial cells, and then form meronts (3) with merozoites that rupture from the host cell (4). These may invade other epithelial cells to undergo several merogonous stages (5), or they may penetrate epithelial cells to produce micro- (6–9) or macrogametocytes (10–12), also typical of enteric Coccidia. After fertilization (13) an unsporulated oocyst is formed which then ruptures from the host cell and is shed in the feces of the snake (14). Rodents are the typical secondary host for the facultatively heteroxenous part of the life cycle. When sporulated oocysts are ingested by a rodent (15), sporozoites excyst in the intestine, cross the gut wall, and become disseminated throughout the dermal tissues of the host, probably via the bloodstream. (16) In these cells, at least 2 asexual generations occur (17–19) followed by the sexual stages (20–22). Following fertilization, thin-walled oocysts are formed (24) and 8 sporozoites develop within a membrane, in the absence of a true sporocyst. These rupture from the host cell containing the oocyst, enter macrophages and/or fibroblasts, and are termed caryocysts (25–27). When eaten by the snake host (28), sporozoites are released from caryocysts and development continues in a manner thought to be identical to that known to occur when sporulated oocysts are ingested.
(Original Figure from Duszynski and Upton, 2010. License: CC BY-NC-SA 4.0.)
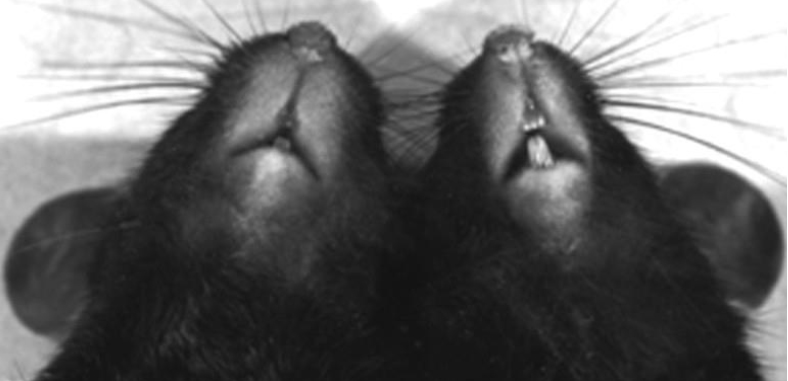
Figure 5D. Photomicrograph of experimentally-infected (left) and control (right) laboratory mice showing the swelling of dermal tissue about 12 days post-infection with 250,000 sporulated oocysts of Caryospora simplex.
(Source: Duszynski and Upton, 2010. License: CC BY-NC-SA 4.0.)
After asexual and sexual multiplication in snake intestinal epithelial cells, typical of that known for other enteric Coccidia, unsporulated oocysts are passed in the feces, but patency may last for months, or even a couple of years; this suggests that either some enteric recycling of asexual stages is occurring or that oocysts are retained deep within host tissues for an unusually long period of time before being released. But the most interesting aspect of the life cycle is what occurs in non-reptile hosts. Rodents are thought to be typical secondary hosts for the non-intestinal phase. Oocysts they ingest undergo excystation, sporozoites cross the gut wall and then become disseminated throughout the dermal tissues. Here, at least 2 asexual generations occur followed by sexual stages. The tissues around the face and neck become edematous (swollen) at this time (Figure 5D). Following fertilization, thin-walled oocysts are formed and 8 sporozoites develop within a membrane, but not a true sporocyst wall. These sporozoites rupture through the thin oocyst wall and enter macrophages and fibroblasts where they become dormant. These modified host cells with dormant sporozoites are termed caryocysts. When eaten by the appropriate snake, sporozoites are liberated from caryocysts and development proceeds in a manner thought to be identical to that which occurs when oocysts are ingested. Although at first glance this type of life cycle may seem unusually complicated, in reality, most developmental stages occurring within the mammalian host appear identical to those occurring in snakes.
Genera Cyclospora Schneider, 1881, Eimeria Schneider, 1875, and Isospora Schneider, 1881 (sensu stricto)
These 3 genera (Figures 6A–F) are considered together because they have mostly identical life cycles, as illustrated in the Eimeria cycle shown in Figure 6A. They differ only in the final morphology of their sporulated oocysts. After sporulation, eimerian oocysts have 4 sporocysts, each containing 2 sporozoites (Figures 6B and 6F–G), cyclosporan oocysts have 2 sporocysts each containing 2 sporozoites (Figure 6C), and isosporan oocysts have 2 sporocysts each containing 4 sporozoites (Figure 6D). Numerous variations may be seen in different species on the surface structures of the oocyst and sporocyst walls (Figures 6B–G).
It is likely that an Eimeria species was 1 of the first protozoa visualized when Antonie van Leeuwenhoek saw what surely were oocysts of Eimeria stiedai Lindemann, 1895 in the bile of a rabbit in 1674. Since the oocyst is the stage that leaves the host, usually in the feces, it is the structure in the life cycle that is readily available to the veterinarian, wildlife biologist, or parasitologist who needs to identify the species without having to kill the host. As a result, about 98% of all Eimeria, Isospora, and Cyclospora species are known only from this 1 life cycle stage, the sporulated oocyst. Eimeria, with perhaps 2,000 named species to date, is the largest apicomplexan genus and may be the most speciose genus of all parasite genera (see Figures 7 and 8), and Isospora has about 250 named species; both have been reported in amphibians, reptiles, mammals, and birds and many Eimeria species (but not Isospora) have been reported in fishes. Fewer than 20 Cyclospora species have been named to date, most in mammals (insectivores, rodents, and primates) and a few in arthropods and reptiles. This genus is best known for 1 species, Cyclospora cayetanensis Ortega et al. 1994, a pathogenic coccidium transmitted by fecal contamination of food (fruits and vegetables) and water, that can cause diarrhea in humans and other primates.
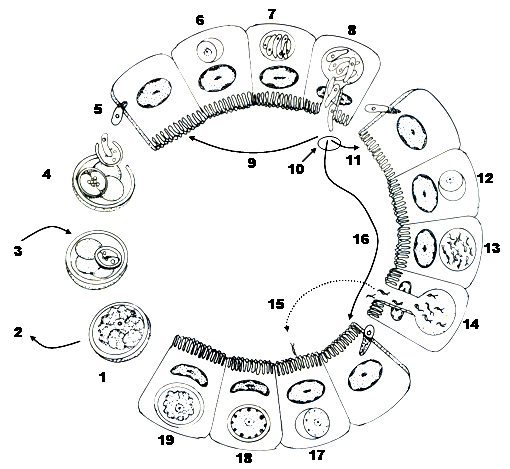
Figure 6A. Homoxenous life cycle of Eimeria species with a direct life cycle (Cyclospora and Isospora species have similar cycles). 1) Unsporulated oocyst leaves the host in its feces. 2) Oocyst needs molecular oxygen, moisture, and a temperature different than the host’s body temperature to sporulate. During sporulation, 4 sporocysts, each with 2 sporozoites are formed. 3) Sporulated oocyst is infective to the next host. 4) Sporozoites are released from sporocysts/oocysts in host’s gut. 5) Sporozoites penetrate host epithelial cells (6) then round up, enclosed in a parasitophorous vacuole to begin merogony. 7) Meront contains several to hundreds to thousands of merozoites. 8) Merozoites destroy host cell and may infect other cells (9) to produce more merogonous stages or (10) last generation of merozoites penetrate enterocytes to begin gamogony. 11) Microgametogony: the merozoite rounds up (12), many bi-flagellated microgametes are produced (13), rupture from their cell (14) and find a host cell with a developing macrogamont (15). 16) Macrogametogony: merozoite rounds up, producing a young macrogamete. After the microgamete penetrates the host cell (15) and fertilizes the macrogamete a young zygote is produced (17). Soon after, wall forming bodies (18) migrate to periphery of cell where they eventually coalesce to form the resistant oocyst wall; once wall is formed and the sporoplasm condenses, (19) the unsporulated oocyst ruptures from the host epithelial cell (1) to be discharged from the host in its feces.
(Source: Duszynski and Upton. License: CC BY-NC-SA 4.0.)
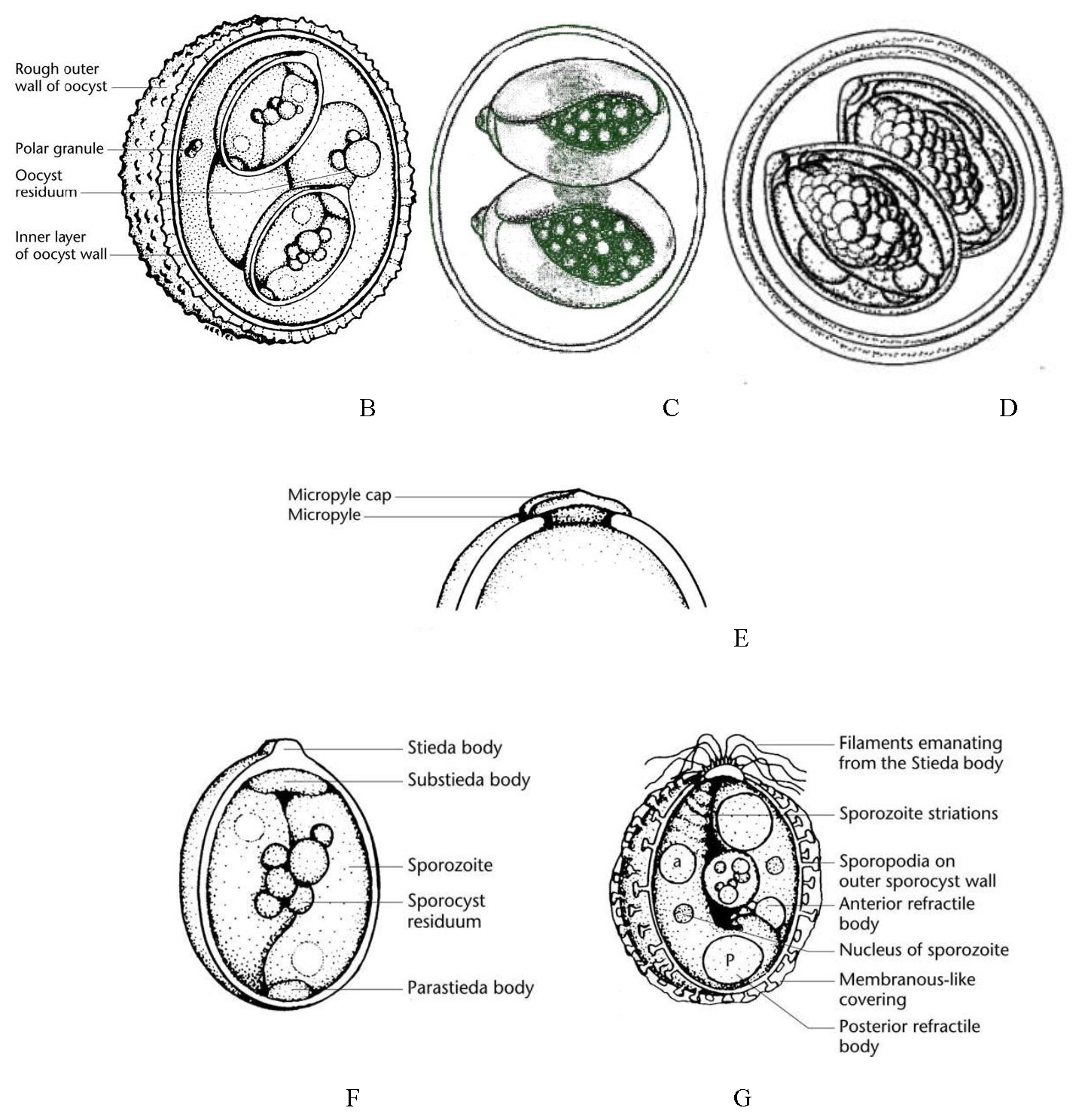
Figures 6B–G. Line drawings of oocyst and sporocyst structures. B) Typical Eimeria oocyst, 4 sporocysts, each with 2 sporozoites. C) Cyclospora oocyst, 2 sporocysts, each with 2 sporozoites. D) Isospora oocyst, 2 sporocysts, each with 4 sporozoites. E) One end of an oocyst with a smooth outer surface and showing other possible structures, a micropyle and micropyle cap. F) Sporulated sporocyst showing major structural features including 2 sporozoites and the Stieda body/substieda body complex. G) Another sporulated sporocyst showing a variety of structural features, some of which may be present on sporocysts of different species.
(Source of all images: Duszynski and Upton, 2010. License: CC BY-NC-SA 4.0.)
The complete life cycle stages of a typical Eimeria species are shown in Figure 6A (see the figure legend for details) and similar life histories are used by Isospora and Cyclospora species. To reiterate briefly, after a sporulated oocyst is ingested by a suitable host, sporozoites excyst and do so by both mechanical (via muscular contractions) and enzymatic (via trypsin or bile salts) digestive processes of the upper gastrointestinal tract in their host. These make the sporocyst and oocyst walls more permeable. Eventually, certain parts of each may be digested, or they may collapse or are broken, releasing their sporozoites so they can penetrate host epithelial cells. Invasion of the host cell is complicated, involving a sequential series of steps including recognition of a host cell, attachment to surface components, formation of a tight junction, entry into the cell (facilitated by organelles of the apical complex), and formation of a parasitophorous vacuole (PV) around the sporozoite (Sam-Yellowe, 1996). Inside its PV, the sporozoite initiates merogony (that is, asexual multiple fission).
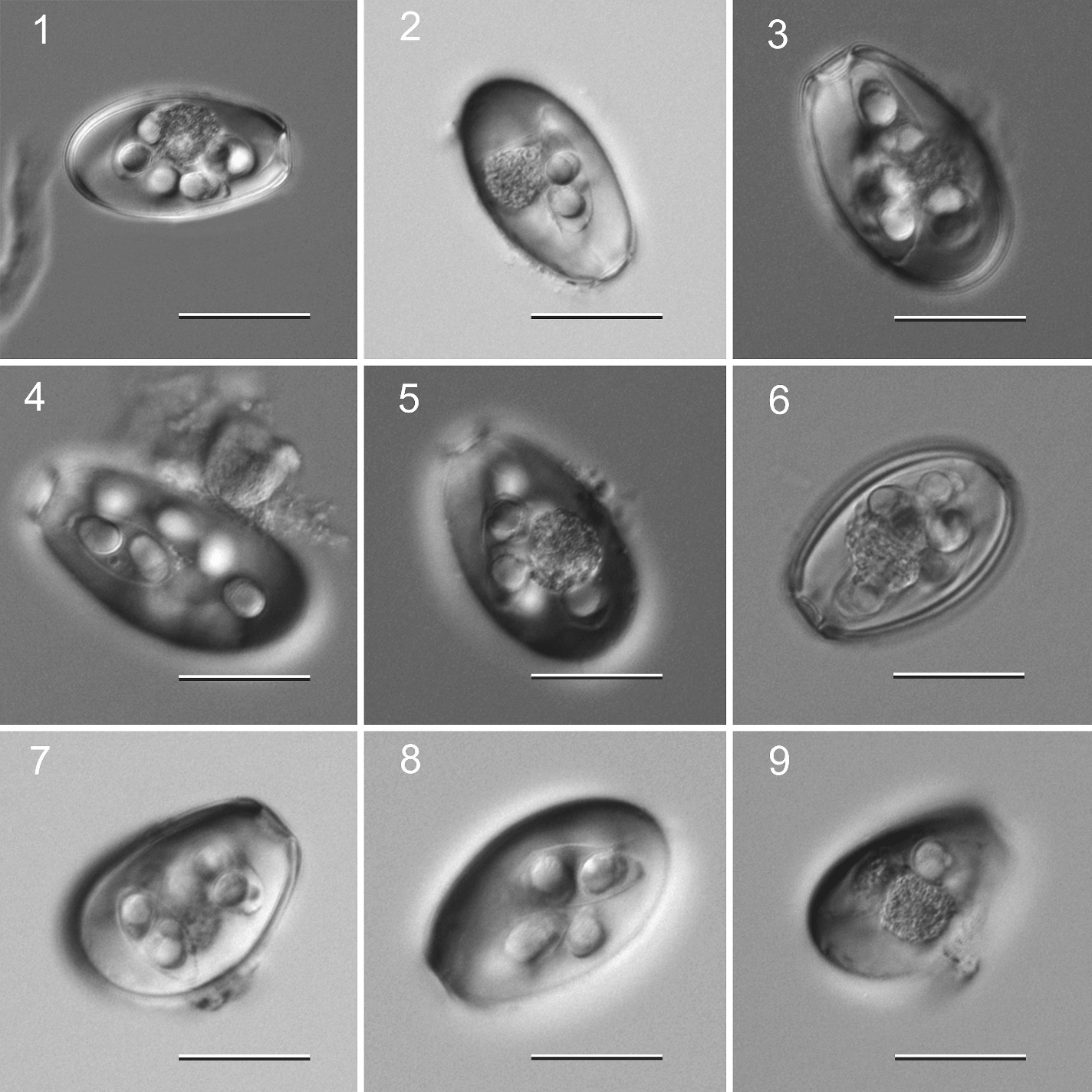
Figure 7. Examples of species of Eimeria from a Mongolian hare Lepus tolai from Mongolia. Scale bar = 25 μm.
(Source: S. L. Gardner, HWML. License: CC BY.)
During merogony, as few as 2, or up to as many as 100,000, merozoites may be formed by each sporozoite, depending on the species. Once mature, merozoites rupture the host cell, each seeking to penetrate a new epithelial cell to begin merogony again. It is believed that each species is genetically programmed for a specific number of merogonous generations. This was first demonstrated by Levine (1940). In this classic paper, he transferred merozoites of Eimeria necatrix from the intestine of 1 chicken to a second, Coccidia-free chicken and showed that the time required for development of oocysts in the second bird was equal to that required in a single host, thus, showing that the length of the life cycle was determined not by increasing resistance of the host, but was inherent in each species of Eimeria. For the few Coccidia species of which we know the actual number of asexual generations, it most often varies from 2 to 4 generations. Whatever the number, tremendous biological magnification of the parasite results from these developmental stages.
When the last generation of merozoites enter host epithelial cells, they develop not into additional meronts, but into gamonts. The vast majority develop into macrogametocytes (macrogamonts) to form uninucleate macrogametes, whereas the remaining merozoites develop into microgametocytes, each of which will undergo multiple fissions to produce thousands of motile, flagellated microgametes, but the precise mechanism that regulates if and when a merozoite will become a macrogamete or microgamete is unknown. Microgametes all are similar in structure with an elongate nucleus, an equally elongate mitochondrion, and 2 or 3 flagella (Scholtyseck, 1979). The nucleus occupies most of the space in the microgamete, which averages 4–7 mm-long. The elongate mitochondrion, about 2–5 mm-long, lies closely adjacent to and often in a groove of the nucleus. When they are mature, microgametes exit their host cell to seek out and penetrate cells with a mature macrogamete, but how microgametes find cells with developed macrogametes inside them, and details of the fertilization process, are unknown and warrant further study. When fertilization does occur, the diploid (2n) condition is restored. Thus, infections with these 3 genera are self-limiting as asexual reproduction does not continue indefinitely.
Family Sarcocystidae Poche, 1913
A second major family in the Eimeriorina, Sarcocystidae Poche, 1913, has 3 subfamilies, Cystoisosporinae Frenkel et al., 1987, Sarcocystinae Poche, 1913, and Toxoplasmatinae Biocca, 1957. All have Isospora-like oocysts in their life cycles with 2 sporocysts, each containing 4 sporozoites, but none of the sporocysts ever have a Stieda body. Instead, their sporocysts have longitudinal sutures that divide the surface into 4 or more plates.
Subfamily Cystoisosporinae Frenkel et al., 1987
Frenkel and others (1987, p. 250) noted, “How we classify the heretofore unthought of cycles and stages is a scientific problem of taxonomy rather than of nomenclature.” Their new taxonomic ideas on these genera with heteroxenous life cycles reflects on the reproductive and transmission strategies of the parasites while maintaining the nomenclature. For this reason, they created 3 separate taxonomic concepts (subfamilies) for the isosporid coccidia without Stieda bodies in the interest of stability, uniqueness, and distinction.
Genus Cystoisospora Frenkel, 1977
Frenkel (1977, p. 620 and 625) erected the genus Cystoisospora to include those mammalian Isospora species with no Stieda body complex in their sporocysts, and with the ability to produce unique monozoic tissue cysts (MZTC) in intermediate or paratenic hosts, and these MZTC stages are a defining character of Cystoisospora species. Earlier, Frenkel and Dubey (1972) discovered the occurrence of tissue cyst stages of 2 intestinal coccidia of cats, C. felis and C. rivolta, in rodent paratenic hosts. They (Frenkel and Dubey, 1972) and others (Dubey, 1975; 1978a; 1978b; Rommel and Zielasko, 1981) also demonstrated that extraintestinal (EIN) stages can occur in the tissues (mesenteric lymph nodes, liver, spleen, lungs, brain, and musculature) of cats and dogs (which are definitive hosts) when fed sporulated oocysts of C. felis and C. rivolta, or C. canis, respectively. When either sporulated oocysts or infected intermediate hosts are ingested, these parasites undergo merogony and gamogony in the intestinal epithelial cells of the carnivore definitive host and, ultimately, they discharge unsporulated oocysts with relatively thick walls (for example, C. felis and C. rivolta of felids; C. canis, C. ohioensis, and C. vulpina of canids). Thus, oocysts of Cystoisospora species look identical to Isospora species except for their ability to infect additional host species (Fayer and Dubey, 1987).
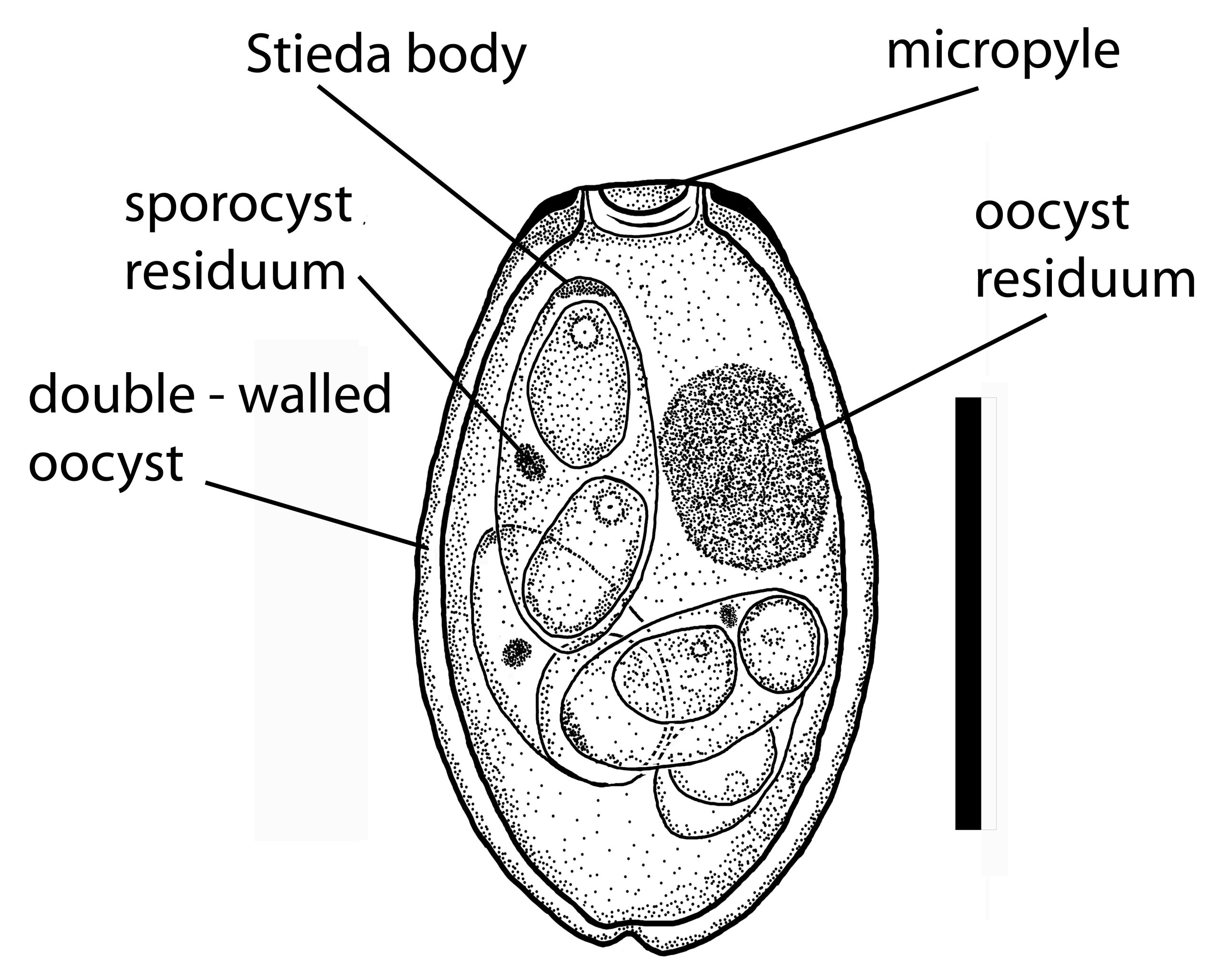
Figure 8. An oocyst of Eimeria gobiensis from a Mongolian hare Lepus tolai in Mongolia.
(Source: S. L. Gardner, HWML, 2009. License: CC BY.)
Subfamily Sarcocystinae Poche, 1913
This is the second subfamily within the Sarcocystidae Poche, 1913 and contains 2 genera (Sarcocystis and Frenkelia). Votýpka et al. (1998), Modrý et al. (2004), and others consider Sarcocystis and Frenkelia as synonyms.
Genus Sarcocystis Lancaster, 1882
Miescher (1843) was the first to see what he called milky white threads (which were actually sarcocysts) in the skeletal muscles of a house mouse in Switzerland, and Huet (1882) saw the first sarcocysts in the muscles of a carnivore, a sea lion that died in the Jardin des Plantes de Paris, France. Lankester (1882) introduced the genus name for these Miescher’s tubules to reflect what he saw, muscle (in Greek, sarco means flesh or muscle) and cyst (in Greek, cyst means bladder or bag), and Blanchard (1885) named the organism Miescheria hueti. Finally, Labbé (1899) transferred this parasite to the genus Sarcocystis. The seminal work by Fayer (1970; 1972) first reported the transformation of bradyzoites from muscle cysts in grackles Quiscalus quiscula into gametocytes and oocysts in cell culture, and this was soon followed by Rommel and colleagues (1972) who described the shedding of sporulated sporocysts from cats after they ingested sarcocyst-infected mutton (also known as Sarcocystis tenella). Thus, the life cycle of all Sarcocystis species is now known to be an obligate, indirect cycle in which the definitive host is a carnivore in which only gametogony occurs, with the release of thin-walled sporulated oocysts or individual infective sporocysts; these stages must be ingested by a suitable intermediate host, in which tissue sarcocysts develop, and only these sarcocysts are infective for the definitive host (but not oocysts/sporocysts) (Figure 9).
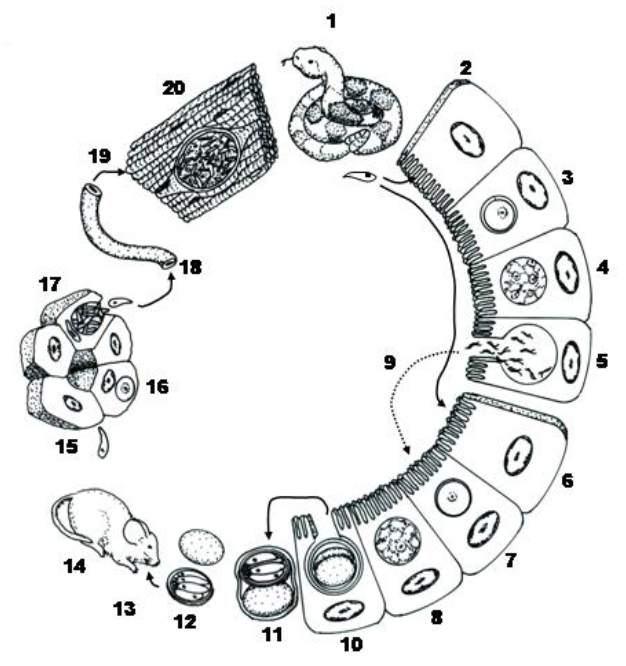
Figure 9. Typical life cycle of a Sarcocystis species with its obligate indirect life cycle. 1) Definitive host ingests infected prey items with sarcocysts in their tissues, bradyzoites are released and penetrate enterocytes of small intestine (2) where they develop directly into micro- (3–5) or macrogametocytes (6–8). After fertilization (5–7), sporogony occurs (8) in the lamina propria and sporulated oocysts are slowly released into the gut lumen fully formed and infective. (10) Oocyst wall is thin, it often ruptures during transit down the intestinal tract releasing 2 sporocysts in the feces, each with 4 sporozoites, rather than intact Isospora-type oocysts. 11) When oocysts and/or sporocysts are ingested by intermediate hosts, excystation occurs in the small intestine, sporozoites penetrate gut wall and enter a variety of extraintestinal tissues (12–14). 15–18) Precystic merogony usually occurs in tissues and merozoites from the last generation to enter the blood and are carried to striated muscles throughout body where they become bradyzoites and initiate sarcocyst formation. 19) Sarcocysts, with thousands of infective bradyzoites (20), are infective to the definitive host when it ingests an infected prey animal.
(Source: Duszynski and Upton, 2010. License: CC BY-NC-SA 4.0.)
Sarcocystis muris Transmission — Learn More
Smith and Frenkel (1978) found sarcocysts in skeletal muscles of some lab mice housed in the same room as cats that had shed sporulated sporocysts of Sarcocystis muris. They noted that cat feces never came in proximity with mouse cages, but they saw German cockroaches (Blatella germanica) in the same room from time to time. To assess the role of B. germanica and the American cockroach (Periplaneta americana) in transmission, cockroaches were exposed to cat feces that contained oocysts/sporocysts of S. muris, Isospora felis, and Toxoplasma gondii. They found that S. muris sporocysts, which remained infectious in cat feces for at least 20 days, were transmitted to mice by P. americana for at least 20 days, and by B. germanica for 5 days post-exposure to infected cat feces.
Dubey and others (2015) published an extensive treatise on Sarcocystis species in humans and other animals and listed 195 names as valid (Dubey et al., 2015, Table 24.1), 49 Sarcocystis species names as invalid (Dubey et al., 2015, Table 24.2), and 83 names (Sarcocystis sp.) that have never received a binomial. Students, and all interested readers, in all disciplines, should use these references when interested in maximizing Sarcocystis species data for any particular host species group.
Subfamily Toxoplasmatinae Biocca, 1957
There are 3 important genera within the subfamily Toxoplasmatinae which need to be mentioned. All of them have somewhat unusual complicated life histories and all of them have Isospora-type oocysts, but they are small and their sporocysts do not have Stieda bodies. An overview of each genus is covered below.
Genus Besnoitia Henry, 1913
Darling (1910) found unusual cysts in an opossum Didelphis marsupialis in Panama and thought the parasite was a species of Sarcocystis, even though he expressed concern with some of the features in the cysts compared to the defining characteristics of the genus. Besnoit and Robin (1912) found a protozoan in France that caused cutaneous and internal lesions in cattle associated with subspheroidal cysts. They also tentatively referred to this organism as Sarcocystis, but did not propose a binomial. Marotel (1912), unaware of Darling’s (1910) paper, discussed Besnoit and Robin’s (1912) work, and wrote, “Nothing similar has been found in animals … and this is why I propose to designate the parasite with the name Sarcocystis besnoiti” (Jellison, 1956). The next year, Henry (1913) reexamined the characteristics of the organism and the nomenclature assigned to it, and used the genus name Besnoitia.
Besnoitia species are obligate heteroxenous Coccidia, similar to those of Sarcocystis species, but they differ from Sarcocystis in 2 unique ways: 1) Oocysts are shed unsporulated by their definitive hosts and have relatively thick walls; and 2) these species can be successfully propagated asexually by mechanical transmission from intermediate host to intermediate host by blood-sucking arthropods. Their life cycles are similar to those of Sarcocystis species because the completion of the sexual cycle in the definitive host is dependent upon ingestion of tissue cysts from a suitable intermediate host—that is, the ability of oocysts to initiate gametogenesis in the definitive host also has been lost. Other details of what little is known about the life cycle of various Besnoitia species are summarized elsewhere (Leighton and Gajadhar, 2001; Dubey et al., 2003; Houk et al., 2011; Charles et al., 2011; and Duszynski and Couch, 2013). Besnoitia is the fifth apicomplexan to be a mammalian tissue parasite, along with Cystoisospora, Hammondia, Sarcocystis, and Toxoplasma. There are now approximately 10 valid species in this genus; the definitive host, which is a carnivore, is only known for 4 or 5 of these species and it is the domestic cat (Felis silvestris catus).
Genus Neospora Dubey et al., 1988
In 1984, a neuromuscular syndrome in dogs that simulated toxoplasmosis was documented by 3 Norwegian veterinarians (Bjerkås et al., 1984), who reported a protozoan causing severe encephalomyelitis in 6 Norwegian pups, but which had no antibodies to Toxoplasma gondii. All dogs originated from 3 litters from a single Boxer female. The pups appeared healthy until 2 months old. Five of these pups had neurological signs for several months, and all 6 were examined at necropsy and diagnosed with encephalitis and myositis with protozoa found in the lesions, including numerous tachyzoites and a few tissue cysts in their brains. Ultrastructural examination of tachyzoites showed them to be similar to those of T. gondii, but with more rhoptries. This confirmed the vertical transmission of this new, unnamed protozoan parasite.
Dubey and colleagues (1988) examined tissue sections and case histories from all dogs and cats that had died of a Toxoplasma gondii-like illness from 1952 to 1987 and were archived at the Angell Memorial Animal Hospital (AMAH), Boston, Massachusetts, the largest hospital for dogs and cats in the United States, which keeps meticulous records of pathology cases. Together, they examined thousands of slides from dogs and cats, and concluded that the syndrome recognized by Bjerkås ad others (1984) was not toxoplasmosis (see the review in Dubey et al., 2017). The records also showed that, in addition to neuromuscular clinical signs, dogs suffered severe disease involving the heart, lungs, liver, and the skin. Dubey and colleagues (1988) found a similar parasite in formalin-fixed tissues from 10 dogs in the United States, named a new genus, Neospora. Neospora caninum Dubey et al., 1988 later became the type species.
A decade later, McAllister and others (1998) firmly established dogs as the definitive host of Neospora caninum and their genus definition included: 1) Tissue cysts in several cell types, but primarily in the neural tissues; 2) a tissue cyst wall up to 4 μm thick, much thicker than Toxoplasma gondii tissue cysts (~ 0.5 μm); 3) numerous bradyzoites, not separated by septa; 4) tachyzoites with numerous electron-dense rhoptries, some posterior to the nucleus; 5) canids (dogs, coyotes, and wolves) as definitive hosts and many intermediate hosts, including dogs, cattle, horses, goats, deer, water buffaloes, coyotes, red foxes, and camels (see also Dubey, 1999; Lindsay and Dubey, 2000); 6) tachyzoites and tissue cysts in both intermediate and definitive hosts; 7) oocysts excreted unsporulated; 8) antibodies to T. gondii not present in infected dogs and the parasites not reacting to T. gondii antibodies in immunohistochemical tests; 9) transmission by carnivorism, transplacental and fecal; and 10) tachyzoites, tissue cysts, and oocysts all infectious to both intermediate and definitive hosts.
Neosporosis — Learn More
Considerable progress in understanding the biology of neosporosis has been made in the last 30+ years, re-sulting in more than 2,000 scientific publications! For the interested reader, Dubey and colleagues (2017) have written a comprehensive, well organized, easily-read book on this subject.
Genus Toxoplasma Nicolle and Manceaux, 1909
There may be several Toxoplasma species in poikilotherms (Duszynski and Upton, 2010), but most parasitologists who work in this area believe there is only 1 species, T. gondii, in mammals, and it has worldwide distribution. Prior to the early 1970s it was thought that T. gondii might be transmitted by blood sucking arthropods, but it is now known that felids are the definitive host. Clinical toxoplasmosis has been reported in virtually all species of warm-blooded animals, including humans, and domestic and wild animals (Dubey and Beattie, 1988; Dubey, 2010). In fact, T. gondii may be the most ubiquitous parasite on Earth because it can be transmitted directly (fecal/oral, including using arthropods as mechanical vectors), transplacentally, and by carnivorism (see Duszynski, 2016, p. 133–140 for a brief review).
Toxoplasma gondii has an indirect life cycle with only felids serving as definitive hosts in which the parasite goes through both asexual and sexual endogenous development in intestinal epithelial cells. All other vertebrate animals that ingest sporulated oocysts are susceptible to infection but, in them, T. gondii forms cysts in cells of virtually any tissue in the body. If these tissue cysts are eaten by another omnivore or non-felid carnivore, the process can be repeated, with the development of tissue cysts in the new host. When cats consume a host animal harboring mature tissue cysts, endogenous development in the gut can be initiated (depending upon the cat’s immune status to T. gondii from a previous infection), and/or bradyzoites from the ingested cysts can go on to develop in the tissues of the cat, too.
Dubey and Frenkel (1972) outlined the sequence of events in the epithelial cells of cats inoculated orally with tissue cysts of Toxoplasma gondii and found 5 new structural stages they designated as types A–D. Interestingly, the feeding of each of the 3 principal T. gondii stages to cats results in different prepatent periods. If chronically-infected mice (characterized by older tissue cysts with bradyzoites) are fed to cats, oocysts can be found in cat feces 3–5 days post-infection (dpi). Cats fed acutely-infected mice (characterized by young tissue cysts with tachyzoites) won’t shed unsporulated oocysts until 5–10 dpi, and cats fed sporulated oocysts usually do not begin to shed oocysts until at least 20–24 dpi.
The mechanisms by which Toxoplasma gondii is transmitted in nature to maintain its ubiquity as an infectious agent still are not completely understood because they are so highly varied. Insects in nature can become infected and, if ingested by mammals or birds, insects may be important transport or paratenic hosts. Wallace (1971) demonstrated the potential of both Musca domestica (common house fly) and Chrysomya megacephala (latrine fly) to be able to transmit sporulated oocysts of T. gondii for at least 24 and 48 hours, respectively, and Periplaneta americana (American cockroach) and Rhyparobia maderae (Madeira cockroach) for up to 12 days post-infection. However, to be of practical interest, it needed to be determined that some of the more prevalent cockroaches were prone to ingest cat feces. Chinchilla and Ruiz (1976) worked with 3 of the most common cockroaches in Costa Rica, P. americana, P. australasiae, and R. maderae, by experimentally showing that both Periplaneta species ate cat feces even in the presence of common foods (for example, dough, sugar, bread, cheese) found in most Costa Rican homes, and that R. maderae showed the greatest tendency to ingest cat feces. Their results suggest that these insects are potential transport hosts for oocysts of T. gondii in cat feces. They also noted that these 3 cockroach species are the most common in city markets, where cats also abound. Also, Smith and Frenkel (1978) found P. americana and, to a lesser extent, German cockroaches (Blatella germanica), transmitted T. gondii oocysts to mice for up to 10 days post-exposure to infected cat feces.
Oocysts of Toxoplasma gondii also can last a long time in the external environment. Frenkel and Dubey (1973) determined that sporulated oocysts suffer little attrition after constant or intermittent freezing at −6 °C, but greater attrition at −21 °C, and that sporulated oocysts survive −20 °C for 28 days, indicating that freezing weather alone does not eliminate oocyst infectivity from soil contaminated by cat feces. Frenkel and colleagues (1975) looked at the effects of freezing and soil storage in Costa Rica and Kansas, United States. In Costa Rica, infectivity persisted for 1 year in 3 shaded sites, 2 moist sites, and 1 relatively dry site in the soil, and in Kansas infectivity lasted up to 18 months, including 2 winters. Frenkel and others (1975) also recovered oocysts from the surface of 1 Musca, several soil isopods, and earthworms. Dubey (1998) looked at the survival of sporulated T. gondii oocysts under defined temperatures, and then tested their infectivity by mouse bioassay. There was no marked loss of infectivity of oocysts stored at 10-, 15-, 20-, and 25 °C for 200 days; oocysts stored at 35 °C were infective for 32, but not at 62 days, those at 40 °C were infective for 9, but not 28 days, those at 45 °C were infective for 1 day, but not for 2 days. Sporulated oocysts remained infective up to 54 months at 4 °C, and no loss of infectivity was seen in oocysts stored for 106 days at −5 °C and −10 °C, and for 13 months at 0 °C.
There have been thousands of surveys around the world looking for oocysts in cat feces and testing blood for antibodies in a variety of in vitro tests, and inspecting tissues for cysts in many other vertebrates, including many carnivores. Dubey (1976) pointed out that even though > 60% of cats in the United States and elsewhere have antibodies to Toxoplasma gondii, only about 1% or less are found to be shedding unsporulated oocysts at any given time. Weiss and Kim (2007) contributed a definitive textbook on the perspectives and methods of T. gondii as a model apicomplexan.
Family Cryptosporididae Tyzzer, 1907
The taxonomy of this group has changed considerably since it was discovered by Tyzzer (1907; 1910) because it possesses features of both coccidians and gregarines. It was initially classified with the Coccidia, but it was found later to be phylogenetically more closely related to Gregarinasina (Carreno et al., 1999; Barta and Thompson, 2006; Kuo et al., 2008). Currently, it is a distinct group of the Conoidasida, on equal status with the Coccidia and the Gregarinasina (Adl et al., 2012).
Genus Cryptosporidium Tyzzer, 1907
Formerly, Cryptosporidium was thought to be a monospecific genus (Tzipori et al., 1980; Tzipori and Campbell, 1981) because of its presumed lack of host specificity, nearly identical life cycle developmental stages (both exogenous and endogenous), and their shared antigenicity (see Figure 8). However, with the advent of gene sequencing and other molecular innovations that tease apart subtle genetic differences, it is now believed there may be at least 30 valid species, and > 50 genotypes, many of which may be mostly adapted to a narrow spectrum of hosts (Lucio-Forster et al., 2010; Osman et al., 2015; Lihua Xiao, personal communication). However, this area of study is still a work in progress and no definitive documentation exists yet regarding the exact number of Cryptosporidium species (Plutzer and Karanis, 2009; Fayer et al., 2010). Many isolates have been classified as “genotypes,” without species definitions (Fayer, 2010) or binomials, and may simply represent cryptic species.
Cryptosporidium species are obligate, monoxenous, intracellular, but extracytoplasmic, parasites. They have been found to infect a wide variety of vertebrate species worldwide, including humans, their domestic food and companion animals, and many species of wild and laboratory animals. Since recognition of the seeming ubiquity of Cryptosporidium oocysts in host feces, searching for them has historically followed 2 paths. First, their oocysts are intentionally sought out in cases of chronic or acute clinical illness, especially in our domesticated and companion animals. Second, general, non-invasive surveys of larger sample sizes of various vertebrate populations have been conducted worldwide to determine prevalence. But prevalence studies rely principally on morphology of the oocysts found, and therein lies the problem. Oocysts are so small, nondescript, difficult to find, and they lack in mensural characters such that they are virtually impossible to use to identify species. At present, fecal flotation, several staining procedures, and immunofluorescence assays of fecal samples are the most commonly-used laboratory techniques for diagnosing Cryptosporidium. Thus, light microscopy (LM) is routinely used for diagnostics; however, it does not allow the identification of species because of the morphological uniformity of the oocysts. Moreover, LM suffers from low sensitivity, because the oocysts: 1) May be shed in small numbers, often under detectable levels; 2) are translucent and small (~ 4–7 μm-wide); and 3) may be confused with yeasts, fungal spores, and/or other structures in fecal samples. Thus, examination using LM requires a trained technician because the oocysts may be overlooked easily, or may be misdiagnosed, and lead to false-positive diagnoses. Moreover, since oocysts are shed intermittently, 1 negative fecal exam may not necessarily mean that the host individual is not parasitized. Therefore, repeated fecal exams should be undertaken when possible.
Oocysts already are sporulated when passed, thus immediately infective. They remain infective in the environment for a long period of time, are resistant to most common disinfectants, and also are able to survive routine wastewater treatment (Fayer et al., 2000; Ryan and Power, 2012). The most common environmental and alimentary sources of Cryptosporidium are water treatment facilities, raw sewage discharge, especially into rivers, wells, ditches, and oceans, where molluscs, oysters, and vegetables become exposed (Meireles, 2010). When sporulated oocysts have been ingested by a suitable host (Figure 10), the infection is usually self-limiting in immunocompetent individuals, but may become acute leading to morbidity and mortality in immunocompromised ones. Therefore, Cryptosporidium species can and do have a great influence on public health.
Cryptosporidium Diagnosis and Genotyping
Due to their small size, intermittent shedding, and limited morphological variation, only molecular and immunological methods can begin to tease apart the subtle sequence or genetic differences between Cryptosporidium species and genotypes. Of particular relevance in evaluating, detecting, resolving, and differentiating the identity of Cryptosporidium species, the following diagnostic and genotyping methods are particularly useful: Polymerase chain reaction (PCR), real time PCR, nested PCR-RFLP, IMS-qPCR, qPCR-MCA assay, enzyme immunoassays, and sequencing of specific genes or regions (Feng et al., 2009; Gao et al., 2013; Homem et al., 2012; Jiang and Xiao, 2003; Lalonde et al., 2013; Leoni et al., 2006; Lindergard et al., 2003; Silva et al., 2013; Xiao et al., 2004). In the case of Cryptosporidium species, several markers or loci are now commonly employed to determine species or genotype differences including, but not limited to, partial and full sequences of 18S rRNA, Cryptosporidium oocyst wall protein (COWP), 70 kDa heat shock protein (Hsp70), glycoprotein 60 (gp60), and actin genes, with partial 18S rRNA gene sequences being the most commonly used marker. Clearly, combining as many of these techniques as possible is much more sensitive in detecting Cryptosporidium-positive fecal samples (Morgan and Thompson, 1998; McGlade et al., 2003; Scorza et al., 2003; Fayer et al., 2006; others) than could be expected under only LM. However, a positive PCR does not provide information on the viability and infectivity of the pathogen. Thus, a combination of methods (LM, TEM, molecular detection, and immunological methods) is recommended and vital, especially in cases where only a few oocysts are present in the feces, or when any doubts are raised regarding the diagnosis, especially in the isolates involved in human outbreaks and/or epidemiological studies.
The diversity demonstrated by Cryptosporidium species is not surprising. Gregarines are ubiquitous, incredibly diverse parasites, with thousands of species so far described and a heterogeneity of life cycle patterns and developmental forms. The recognition of Cryptosporidium affinities with this group helps to explain the increasing numbers of novel genotypes that are being discovered and emphasizes that the specificity of environmental detection procedures for Cryptosporidium could be compromised by cross-reactivity with gregarine protozoa that are ubiquitous in freshwater environments (Bull et al., 1998; Hijjawi et al., 2002; Tenter et al., 2002).
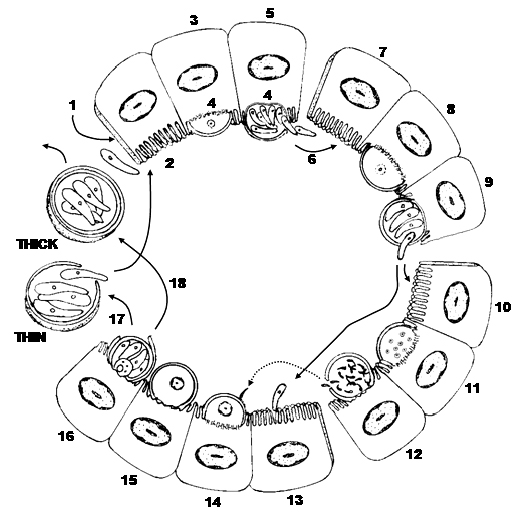
Figure 10. Direct life cycle of a Cryptosporidium species. 1) Ingestion of sporulated (thick-walled) oocyst (4 sporozoites) with contaminated food and/or water. 2) Sporozoites excyst from oocyst and penetrate the microvillus layer of epithelial cell and become enclosed by a thin layer of host cell cytoplasm and membranes (3). 4) A desmosome-like attachment organelle and folding of the parasite membranes develop at the interface between parasite and host cell cytoplasm. 5) Merogony forms 8 merozoites in Type I meront. 6) The meront ruptures the host cell releasing merozoites, which penetrate new host cells (7) forming Type II meronts (8). 9 and 10) Type II merozoites enter other epithelial cells to become microgametocytes (11) that undergo multiple fission (12) to produce ~16 non-flagellated microgametes. (13) Most Type II merozoites penetrate epithelial cells enlarging into a macrogametocyte/macrogamont to become a macrogamete (14). 15) Cells with macrogamonts are penetrated by microgametes which penetrate a macrogamete to form a zygote. 16) Sporogony occurs releasing sporulated oocysts into the environment of the intestinal lumen and the feces. About 20% of oocysts fail to form an oocyst wall (17) and only a series of membranes surround the sporozoites. Sporozoites from these thin-walled oocysts are thought to excyst within the gut and infect new epithelial cells (1 and 2). The remaining 80% of thick-walled oocysts exit the host in the feces to potentially contaminate food and water of future hosts.
(Source: Duszynski and Upton. License: CC BY-NC-SA 4.0.)
A better understanding of the developmental biology of Cryptosporidium in its host can now be achieved by a more comparative approach with what is known of some higher gregarines. This applies to the parasite’s relationship with its host cell and whether Cryptosporidium’s epimerite-like feeder organelle obtains nutrients in a way that is truly analogous to myzocytosis, as utilized by many gregarines, through which host cell contents are obtained. In this respect, it is interesting that the feeder organelle has been observed in extracellular stages in a biofilm environment and thus may be able to acquire nutrients in such a host cell-free environment (see Koh et al., 2014).
Discussion, Conclusions, and Difficulties of Working with Apicomplexa
Species Identifications
Accurate species identification is fundamental to every biological investigation. Taxonomists who work with the Coccidia face numerous challenges when defining new species because these parasites undergo a sequential series of structural changes, both inside (endogenous) and outside (exogenous) their host species. Endogenous developmental stages (multiple stages of merogony, merozoites, micro- and macrogametocytes, developing zygotes/oocysts) exhibit sequential structural changes and to find and measure them requires killing the host. Sporulated oocysts outside the host have been studied the most, historically, because they are resistant to environmental extremes, can be collected by non-invasive means (fecal collection/preservation), and usually can be maintained for long periods of time. Unfortunately, however, oocysts have only a small suite of qualitative and structural characteristics that are quantifiable in the Eimeriidae, but especially in the Cryptosporididae. Generally, the identification of Eimeria, Isospora, Caryospora, and other species in the family is based primarily on their oocyst features without other supporting information (Jirků et al., 2009). Thus, to date, ~ 2,000 nominal species of these genera have been described, with ~ 98% of them identified only by their oocyst’s morphology (Asmundsson et al., 2006; Ghimire, 2010). The morphology of oocyst structures both within and between host species can be quite diverse to the point that it becomes confusing and is sometimes difficult to distinguish species based entirely on morphological features. Thus, morphology alone is no longer sufficient to confidently identify many coccidian species, especially those in genera with very small oocysts and sporocysts. These identifications should be supplemented by multiple data sets with information collected from, but not limited to, site of sporulation (endogenous versus exogenous), information on the location and sizes of some or all of the endogenous developmental stages, and sequence data to conduct phylogenetic analyses that will allow the investigator to more robustly assign a parasite to a group, genus, or even species (for example, see Merino et al., 2008; 2009; 2010).
When Do Oocysts Become Sexual?
There is only limited information on sexual differentiation in endogenous and/or exogenous life cycle stages. Canning (1963), Klimes and colleagues (1972), and others (Jeffers, 1978; Cornelissen et al., 1984; Cornelissen and Overdulve, 1985) showed that merozoites were sexually differentiated. This may happen either between the sporozoite and the first generation meront/merozoites or the first generation merozoites could be sexually undifferentiated, and gene expression is responsible for the formation of either male or female type second generation meronts/merozoites. Cornelissen and colleagues (1984) called these merozoites macro- or microgamontoblasts. Microgamontoblast merozoites were reported to have only a few granules of polysaccharide reserves and their nuclei lack nucleoli, while those giving rise to macrogamonts had abundant coarse granules of polysaccharide and the nuclei each have a conspicuous nucleolus. Both gamontoblasts contained the haploid amount of DNA and none has been found to be synthesizing DNA.
There is no good evidence that fertilization of a macrogamete is a necessary stimulus to form the oocyst wall, but in oocysts which do sporulate, the zygote sporoplasm is the only stage to possess a diploid nucleus. During the first nuclear division of sporogony, chromosome reduction occurs in a single meiotic division, whereas 2 subsequent nuclear divisions within the zygote are thought to be mitotic. Thus, sporozoites in each sporocyst, as products of a meiosis, would be genetically identical; since infection with either a single sporocyst, or even a single sporozoite, produces viable oocysts in the right host, suggesting that sexual differentiation occurs at a stage in the life cycle later than sporogony. This makes the 2 sexually undifferentiated sporozoites in each sporocyst the basic unit of propagation. It is clearly the advantage of the parasite to remain sexually undifferentiated until it is well established in the host, thus avoiding the possibility of unsuccessful infections due to the loss of sporozoites of the opposite type (Lee et al., 1977).
One sporulated oocyst doesn’t necessarily represent a population of genetically identical organisms because it may contain recombination characters from 2 different parental lines. Once ingested and the sporozoites are released, they penetrate epithelial cells and merogony begins. The question then arises, when and where does sexual differentiation occur? The sexuality of individual sporozoites was debated throughout the 1960s and 1970s, but sufficient work was done to indicate they most likely are bisexual (Haberkorn, 1970; Shirley and Millard, 1976; Jeffers, 1978; Cornelissen et al., 1984; Cornelissen and Overdulve, 1985). That is, sexual differentiation is influenced by environmental stimuli responsible for their expression, but the exact nature of exogenous stimuli is unclear. This demonstrates that true clones of Eimeria can be established only from individual sporozoites or sporocysts. If sex were determined by genetic factors which segregate during zygotic meiosis, individual sporocysts would contain sporozoites of like sex and would be incapable of producing a complete infection that could produce zygotes.
Oocyst Production
The time between when a suitable host ingests a sporulated oocyst and when oocysts leave that host in its feces is termed the prepatent period. During this interval, which can vary from 3 to 10 days (or more), no oocysts are found in the feces because only merogony and the beginning of gamogony are occurring in the host. The time interval during which oocysts are discharged from an infected host is termed the patent period and lasts until all the fertilized and unfertilized macrogametes have been released from their host cells. Both time periods vary between host and coccidian species and are dependent on many factors including: Coccidian species, number of oocysts ingested, number of endogenous stages for that species, depth within the tissues where merogony, gamogony, and fertilization occur concurrent infection with other parasites, host age, nutritional and immune status, and other ecological and physiological factors that are not yet understood.
Once outside the host, the oocyst must sporulate in many species before it is infective to another suitable host. The presence of oxygen, moisture, shade (direct exposure to ultraviolet radiation—sunlight—will kill oocysts quickly) and, generally, a temperature less than the body temperature of the host, all are necessary for oocyst survival. If these conditions are met, complete sporulation occurs and the fully formed oocyst and sporocysts are resistant to environmental extremes, and the sporozoites therein are immediately infective to the next suitable hosts that may ingest them. Each oocyst has a suite of structural characters, unique to its species, that can help the experienced taxonomist distinguish one species from the next in many instances. Unfortunately, because this suite of characters is so small, sometimes sporulated oocysts from different host species look very nearly identical in size and structure and may not be easily or reliably differentiated by morphological features alone. In these instances, life history information (for example, tissue stages as in Choleoeimeria) and molecular techniques (such as gene sequencing followed by phylogenetic analysis) is necessary to assist in final identification of the parasite under scrutiny.
Survival of Oocysts
Our understanding of the survival of oocysts in the external environment and the mechanisms by which they reach an appropriate definitive host is minimal and requires additional study. Moisture, temperature, and direct exposure to sunlight all influence the ability of oocysts to sporulate in the external environment (or not), but the interactions of these and other factors (for example, mechanical vectors such as invertebrates) are not well understood. In general, oocysts sporulate more rapidly at higher temperatures and slower at lower temperatures; exposure to temperatures less than 10 ºC or greater than 50 ºC is lethal to unsporulated oocysts. Between these extremes, the sporulation of oocysts in a field-collected fecal sample is dependent on at least the following factors: 1) The parasite species, 2) the time and temperature between collection and arrival of the sample at the laboratory, 3) the medium in which the sample was stored, 4) the amount of molecular oxygen available to the stored oocysts, and 5) the concentration of oocysts in the sample. Under optimal laboratory conditions, sporulation of oocysts from mammals occurs best between 20 °C and 25 °C, but this will vary among vertebrate classes (Duszynski and Wilber, 1997). Interestingly, a few oocysts of some Eimeria species (normally, 4 sporocysts each with 2 sporozoites) can be induced to change into Isospora-like oocysts (2 sporocysts each with 4 sporozoites) when fresh, unsporulated oocysts are first heated to 50 ºC for 30–60 seconds before incubation at 25 °C for a week (Matsui et al., 1989).
Williams and colleagues (2010) reported that, once sporulated, oocysts of some species remain viable and infective in 2% aqueous potassium dichromate (kills bacteria, prevents putrification) at 4–5 ºC for up to 24 years! In their natural external environment, oocysts remain viable and infective from as little as 49 days up to 86 weeks, dependent upon the species and the interplay of abiotic and biotic environmental parameters.
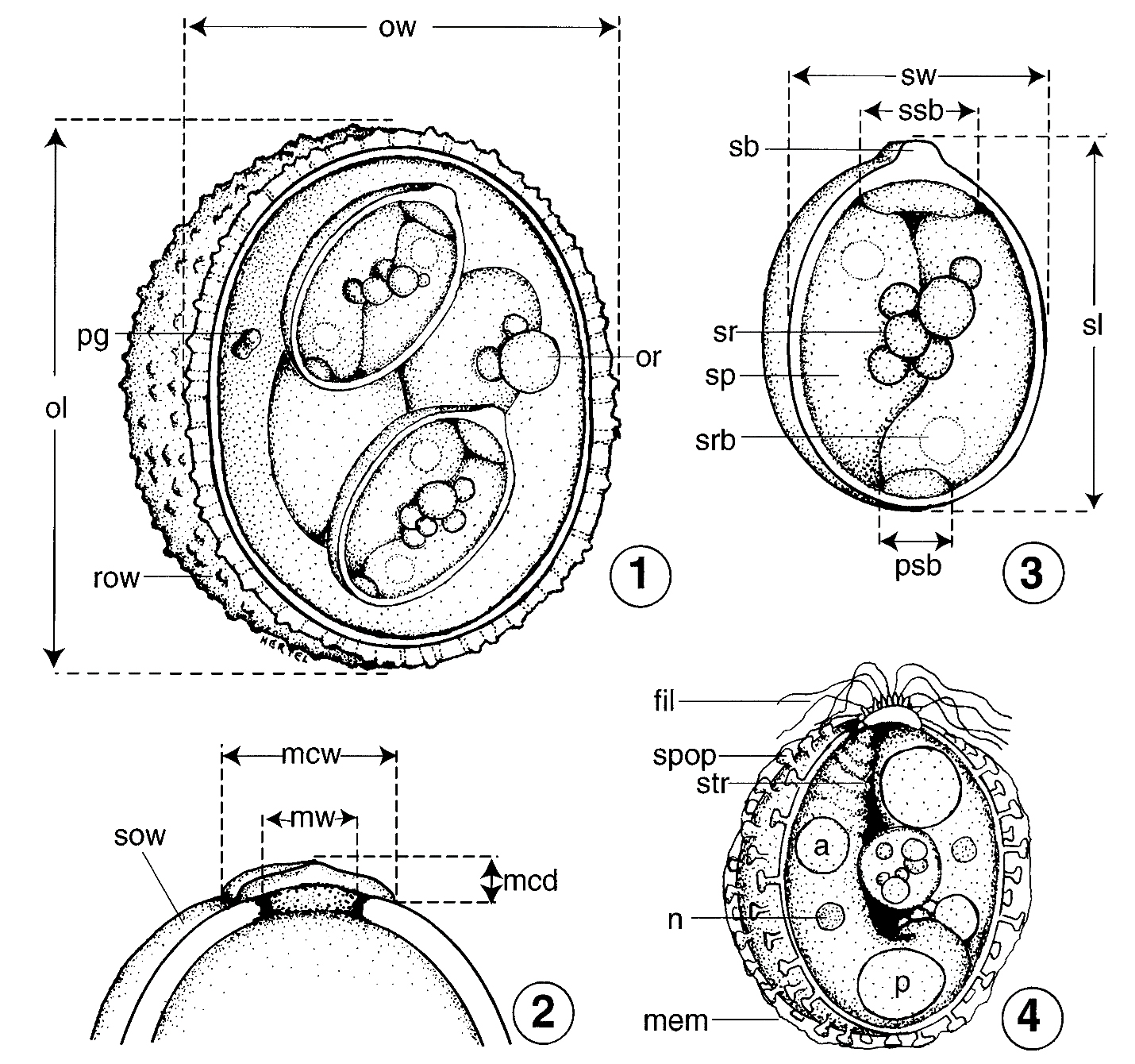
Figure 11A–D. Line drawings of the parts of sporulated oocysts (Eimeriidae: Eimeria, Isospora, et al.) that should be measured and carefully documented when submitting a new species description for publication. A) Sporulated oocyst of an Eimeria sp., drawn in optical cross section, showing essential structural parts that should be measured/documented in the species description: ow, oocyst width, measure the widest part when the oocyst is in good optical cross section under oil immersion; ol, oocyst length; pg, polar granule, note shape and size; or, oocyst residuum, note shape, structure, size, and whether or not it may be membrane bounded; row, rough outer wall, note this feature, if present, as well as its thickness relative to the inner wall (if present). B) The top of an oocyst that has a micropyle, micropyle cap, and a smooth, 1-layered wall: sow, smooth outer wall; mw, width of the micropyle; mcw, width of the micropyle cap; mcd, depth (= height) of the micropyle cap. C) Composite sporulated sporocyst (hypothetical) from an oocyst of Eimeria sp., drawn in optical cross section, and enlarged to show detail: sw, sporocyst width, measure the widest part when the sporocyst is in optical cross section under oil immersion; sl, sporocyst length; sb, Stieda body; ssb, substieda body, measure width and note relationship to sb (for example, 2 × wider); psb, parastieda body, measure width and height (if possible); sr, sporocyst residuum, note shape, structure, size, and whether or not it may be membrane bounded; sp, sporozoite, note any peculiar or unique features; srb, sporozoite refractile body, note size, number, and relative locations in sp. D) Composite sporulated sporocyst (hypothetical) showing a number of unique structural features that may be present in/on the sporocysts/sporozoites of certain eimeriid species: fil, filaments emanating from the area of the Stieda body; spop, sporopodia extending from the outer surface of sporocyst wall; mem, membranous-like covering sometimes associated with sporopodia; n, a nucleus sometimes is visible within sporozoite; str, sporozoites sometimes have striations at their anterior end; although some sporozoites have only 1 refractile body (Figure 9C), others have both anterior (a) and posterior (p) refractile bodies as shown here.
(Source: Original by L. A. Hertel; adapted from Duszynski and Wilber, 1997. License: CC BY-NC-SA 4.0.)
Other Means of Transmission?
The role that naturally occurring soil (for example, mites, ticks, earthworms, and so on) or household organisms (such as house flies and cockroaches) can serve as mechanical vectors has been little studied, but it is known that in many instances invertebrates can be important contributors to the continuation of coccidian life cycles. In Hepatozoon species we know that many invertebrate species (such as mites, ticks, and so on) serve as the definitive host while a vertebrate becomes the intermediate host, and gamonts of the parasite in its red blood cells must be ingested by the intermediate host for the cycle to complete. We know that Besnoitia species can be propagated asexually by mechanical transmission from intermediate host to intermediate host by blood sucking arthropods. Cockroaches are known to transmit oocysts/sporocysts of Sarcocystis species to mice in which sarcocysts can and/or will form. Goodwin and Waltman (1996) demonstrated that darkling beetles (Alphitobius diaperimus) could transmit sporulated oocysts of Eimeria species to chicks inoculated with beetle homogenates (also see Markus, 1974; 1980; Clubb and Frenkel, 1992).
It has been demonstrated experimentally that at least a few bird and mammalian Eimeria may form extraintestinal tissue stages (Carpenter, 1993; Mottalei et al., 1992). Apparently, sporozoites excyst from oocysts ingested by these hosts, infect cells in various places in the body and become dormant. The infected host may or may not be the ‘normal’ host for that Eimeria species; if the host with such tissue stages is eaten by the appropriate host, these dormant sporozoites become active, infect enterocytes (which are intestinal epithelial cells) and initiate their typical life cycle. It is not known if such a cycle is functional in natural communities. And, of course, some Caryospora species are facultatively heteroxenous. Cystoisospora, Besnoitia, and Toxoplasma species form tissue cysts in intermediate hosts that can continue these cycles where those intermediate hosts are ingested.
Finally, another area that needs further study is to determine the mechanisms of how Eimeria overwinter in hibernating animals and the importance of these mechanisms to their maintenance in natural populations.
Ubiquitous, Neglected, and Complex: Untapped Biodiversity
The number of species of eimeriid coccidia is potentially staggering because these parasites have been found to infect all vertebrate and some invertebrate species that have been sampled for them. Unfortunately, most parasite surveys of vertebrates have concentrated only on their helminth and/or arthropod companions and largely have ignored their Eimeria (and other protozoan) parasites. For example, looking at the 5 classes of vertebrates we know only the following about their coccidia to date:
Amphibia (Frogs, Toads, and Salamanders)
This class has 3 orders, 56 families, 464 genera, and 6,009 species. Only 14 of 56 (25%) extant families, 28 of 464 (6%) genera, and 45 of 6,009 (< 1%) species have ever been examined for Coccidia. From these surveys 89 identifications were made. These include 52 Coccidia species described and given binomials: 38 Eimeria, 11 Isospora, 2 Goussia, and 1 Hyaloklossia species. In addition, 37 additional names appeared that researchers believe are not valid including: 10 species inquirendae (which are species of doubtful identity), 22 incertae sedis (which have been placed in an uncertain taxonomic position), and 5 nomen nuda (which are nude names without formal descriptions) are entered into the literature (Duszynski et al., 2007).
Aves (Birds)
No definitive summary exists yet for Eimeria or other Coccidia genera from the 2 superorders and 29 orders of about 10,000 extant bird species, but it is known that there are many Eimeria and Isospora species already described (along with at least 6 other coccidian genera) and that some of these species, especially some eimerians from chickens and turkeys, can be exceptionally pathogenic to their hosts.
Mammalia (Mammals)
There are about 5,416 mammal species organized into 1,229 genera in 53 families placed into 29 orders (Wilson and Reeder, 2005). It is noteworthy that 13 of 29 (45%) orders have been looked at in detail for their Coccidia including:
Soricomorpha (Insectivores)
In the insectivores, 4 of 7 (57%) families, 19 of 66 (29%) genera, and 37 of 428 (9%) species have been examined for their Coccidia. From these surveys, 120 Coccidia species were described including: 48 Eimeria, 22 Isospora, 5 Cyclospora, and 45 species inquirendae including Coccidium, Cyclospora, Eimeria, Gousseffia, Isospora, and “Coccidia” species (Duszynski and Upton, 2000).
Primates (Monkeys)
Only 7 of 13 (54%) families, 14 of 60 (23%) genera, and 18 of 233 (8%) species have been examined for their Coccidia. From these surveys 28 Coccidia species were described including: 7 Eimeria, 8 Isospora, 1 Cyclospora, and 12 species inquirendae (Duszynski et al., 1999).
Scandentia (Tree Shrews)
Tree shrews all are placed in 1 family. Only 2 of 5 (40%) genera and 4 of 19 (21%) species in this family have been examined for coccidians. From these surveys only 4 Eimeria species have been described (Duszynski et al., 1999).
Chiroptera (Bats)
Only 6 of 17 (35%) families, 37 of 177 (21%) genera, and 86 of 925 (9%) species have been examined for their Coccidia. From these surveys 39 Coccidia species were described including 31 Eimeria and 8 species inquirendae (Duszynski, 2002).
Lagomorpha (Rabbits)
When compared to other mammalian orders, the Lagomorpha is not diverse and contains only 2 extant families, but even with such a tractable group not much is known about their coccidian parasites, except for just a few species. Although species in both extant families have been studied (a little), only 5 of 12 (42%) extant genera and 25 of 96 (26%) species have been examined. From these surveys, 87 coccidia species were described including 3 Besnoitia, 3 Cryptosporidium, 73 Eimeria, 2 Isospora, 5 Sarcocystis, and Toxoplasma gondii, and 33 species inquirendae (Duszynski and Couch, 2013).
Marsupialia (Marsupials)
In most earlier classifications of mammals (for example, Nowak, 1991) all marsupials were placed in a single order, but results from molecular and genetic research tools in the last 15 years have directed mammalogists to partition them into 7 orders within 2 superorders: Ameridelphia (Didelphiamorphia, Microbiotheria, Paucituberculata), the American marsupials, and the Australidelphia (Dasyuromorphia, Diprotodontia, Notoryctemorphia, Peramelemorphia) the Australian marsupials (Wilson and Reeder, 2005). Duszynski combines the parasite data for these 7 orders into the original Marsupialia, within which there are 21 families, 92 genera, and 331 species. From all pertinent surveys 154 Coccidia species are named including: 1 Besnoitia, 6 Cryptosporidium, 56 Eimeria, 1 Isospora, 11 Klossiella, 10 Sarcocystis species, Toxoplasma gondii, and 68 species inquirendae from 85 of 331 (26%) marsupial species examined. These species are found in 14 of 21 (67%) families examined for coccidian parasites, in 45 of 92 (49%) genera, and in only 85 of 331 (26%) marsupial species that have been examined for coccidian parasites (Duszynski, 2016).
Carnivora (Carnivores, Cats, and Dogs)
This order is separated into 2 suborders, Caniformia (9 families) and Feliformia (6 families), and these 15 families have 126 genera and 287 species (Wilson and Reeder, 2005). There are about 207 valid coccidian species named including 5 Besnoitia, 1 Caryospora, 10 Cryptosporidium, 1 Cyclospora, 53 Cystoisospora, 39 Eimeria, 3 Hammondia, 6 Hepatozoon, 9 Isospora, 1 Neospora, 78 Sarcocystis, and Toxoplasma gondii. There also are about 483 incompletely named species (species inquirendae) recorded from carnivores that fit taxonomically into these taxa as genus names only, or less. These species are found in 11 of 15 (73%) families, in 30 of 126 (24%) genera, and in only 48 of 287 (17%) carnivore species that have been examined for coccidian parasites.
Rodentia (Mice, Rats, Squirrels, etc.)
There are 33 families, 481 genera, and 2,277 extant species of rodents (Wilson and Reeder, 2005). Although there is no group-by-group summary to date, it is estimated that only about 15% of all rodent species have been surveyed for Coccidia. From these surveys about 450 Eimeria and Isospora species have been described (Duszynski and Upton, 2001).
Remaining 15 Mammalian Orders
No definitive summaries exist to date although some Coccidia species in 8 genera have already been described from a few of their species.
Pisces (Fish)
No definitive summary exists yet for the 32,500 extant fish species although many coccidian species in at least 6 genera already have been described (unpublished data).
Reptiles
Snakes
Only 6 of 17 families (35%), 110 of 457 genera (24%), and 208 of 3,108 snake species (7%) have been examined for their coccidia. From these surveys, 302 coccidia species were described including 52 Caryospora, 2 Cryptosporidium, 4 Cyclospora, 66 Eimeria, 7 Isospora, 22 Sarcocystis, 1 Wenyonella, 2 Tyzzeria, and 148 species inquirendae, the latter including 3 additional genera (Dorisiella, Globidium, and Pythonella) (Duszynski and Upton, 2010).
Turtles
The order Testudines is separated into 2 suborders, Cryptodira (11 families) and Pleurodira (3 families), and these 14 families have 96 genera and 351 species (Uetz, et al., 2018). Surprisingly, at least 1 species in 10 of the 14 (71%) families has been examined for coccidian parasites, but only 30 of 96 (31%) genera and 61 of 351 (17%) turtle species have been examined for Coccidia and 100 Coccidia species in 7 genera are known: 2 Caryospora, 66 Eimeria, 3 Isospora, 1 Sarcocystis, and 28 species inquirendae (4 Coccidium, 1 Caryospora, 9 Cryptosporidium, 5 Eimeria, 1 Manotella, and 8 Sarcocystis species [?]) (Duszynski and Morrow, 2014).
Alligators
The order Crocodylia includes 27 species of alligators, caimans, crocodiles, and gharials. Duszynski and colleagues (2020) reported the blood and intestinal apicomplexans know to date from these reptiles and concluded that 17/27 (63%) had 16 apicomplexan species unique to them including: 8 Eimeria, 1 Haemogregarina, 4 Hepatozoon, 2 Isospora, and 1 Progarnia species; they also reported an additional 46 apicomplexan-like forms that were considered species inquirendae that await further study.
Lizards
No definitive summary exists yet for all lizards, although many apicomplexans in 8 genera already have been described (unpublished data). This is now a work in progress.
There are approximately 62,150 extant vertebrate species known on Earth and, to date, there is comprehensive, systematic survey data on 16 vertebrate groups, (amphibians, 13 mammalian orders, snakes, turtles, crocodiles) which comprise 11,787 species, but only 634 (5.3%) of these species have been examined for Coccidia and from them about 1,146 species have been named in the literature or about 1.8 Coccidia species per host species examined. Given that some host species (for example, chickens, rabbits, and others) have 10 or more Eimeria species that may be unique to them, and that even domestic animals, whose parasites have been studied for decades, have had new Eimeria species described from them recently, it is clear that only a fraction of the number of Eimeria (and other coccidia) species that occur in vertebrates have been described to date. Using numbers that are now out of date, Levine (1973) estimated that more than 45,000 species of Eimeria would be found if all vertebrate species were examined. This is a gross underestimation, but it points to the urgent need for more work in this area, especially given the alarming rate of habitat destruction and vertebrate species extinctions occurring worldwide. If we assume, conservatively, that every vertebrate species on Earth is host to (minimally) 2 coccidia species unique to it, we could expect to find at least 124,300 total coccidia species. The 1,800 or so Coccidia species currently known is only 1.4% of the number of species that likely exist in Earth’s vertebrates. In other words, 98.6% (or more) of the coccidian parasites of vertebrates are yet to be discovered! Clearly, there is lots of work to be done.
Ubiquitous, Neglected, and Complex
Specificity
Eimeria species demonstrate both site and host specificity, but to somewhat different degrees. The majority of species for which endogenous development is known undergo development within certain cells of the gastrointestinal tract, but not all species are found in this location. Eimeria stiedai undergoes development in epithelial cells of the bile duct and parenchymal cells of the liver of rabbits. Other species have been found to develop in cells of the gallbladder (goat), placenta (hippopotamus), epididymis (elk), uterus (impala), genitalia of both sexes (hamsters), bile duct (chamois), liver parenchyma (wallaby), and pyloric antrum (kangaroo) (Duszynski and Upton, 2001). Hepatozoon species develop in the blood of vertebrates and in arthropods, Klossiella species develop in kidney epithelial cells, and Cystoisospora, Sarcocystis, Besnoitia, and Toxoplasma have heteroxenous life histories. Once within their specific organ system of choice, Eimeria species seem to be limited to specific zones, specific cells within that zone, and specific locations within those cells. Thus, 1 species may be found only in the middle third of the small intestine and another only in the cells of the cecum. Within their specific region 1 species may be found only in cells at the base of the crypts of Lieberkühn, a second species in epithelial cells along the villi, and a third species in endothelial cells of the lacteals in the villi. Some species develop below the striated (microvillus) border of endothelial cells, but above the nucleus, others below the nucleus and a few within the nucleus. And a few other species or genera associate closely with the brush border of the epithelial cells and may even be extracytoplasmic (for example, Cryptosporidium species).
The degree of host specificity seems to vary from host group to host group; it’s been studied best in mammals, and to a lesser degree in birds, especially domesticated stock or flock animals. Eimeria species from goats cannot be transmitted to sheep and vice versa (Lindsay and Todd, 1993), but Eimeria from cattle (Bos) are found to infect American bison (Bison). Eimeria species from certain rodents (Sciuridae) seem to cross host generic boundaries easily (Wilber et al., 1998), whereas other rodent species (Muridae) may cross species, but not genus boundaries (Hnida and Duszynski, 1999). In the Lagomorpha, 6 of 17 (35%) Eimeria species reported from cottontails (Sylvilagus spp.) are experimentally infective for the tame rabbit (Oryctolagus cuniculus). Similarly, some species from gallinaceous birds can be transmitted only to congeners, whereas others can be cross-transmitted between genera. One species has even been reported to cross familial lines, but this seems rare (De Vos, 1970). It also is known that Eimeria separata Becker and Hall, 1931, from rats will infect certain genetic strains of mice and that genetically altered or immunosuppressed mammals are susceptible to infection with Eimeria species to which they otherwise might be naturally refractory. Thus, numerous biotic interactions, particularly the genome of both parasite and host, must contribute to the host specificity, or lack thereof, attributed to each Eimeria species.
Significance in Biomedical Research
All members of the protozoan phylum Apicomplexa are obligate intracellular parasites. In addition to the Eimeria, many of their closely related cousins (for example, Isospora, Sarcocystis, Tyzzeria, and others) can cause economically important diseases in domesticated, and sometimes wild, animals. Other related forms in the phylum (for example, Toxoplasma, Cyclospora, Cryptosporidium, and Plasmodium) cause human disease in hundreds of millions of people worldwide. Classical genetic studies have been limited by the intracellular habitats of all these organisms and/or by the complex life cycles of some of them. However, development of pulsed-field electrophoresis, DNA sequencing, PCR, and related techniques has allowed good progress in understanding of the genomes of these parasites. In fact, the complete genome sequence of the most pathogenic human malaria parasite, P. falciparum, is now known (Gardner et al., 2002).
Recently, use of some of these molecular techniques has shown that a number of apicomplexan parasites (Eimeria, Plasmodium, and Toxoplasma species) have 2 extrachromosomal DNAs: 1) A small mitochondrial genome and 2) a unique 35 kb circular DNA. Sequencing and other molecular data suggest that the 35 kb DNA may be related to plastid DNA (plDNA). This plDNA should be of keen interest to researchers because its true origin, cellular location, function within these parasite cells, and relation to their nuclear genomes, are still a mystery. The exciting appeal for studying the plDNAs is their potential as specific targets for chemotherapeutics: Potential “silver bullets” to control the undesirable parasites of humans and their domesticated animals that reside within the Apicomplexa. If the sequence of plDNA genes differs significantly from the genes for similar functions in their hosts, then such a function-mediated gene may prove an ideal target for development of chemotherapeutics that could be efficacious and nontoxic, while not inducing resistance. If plDNAs prove to be a common or universal feature of members of the Apicomplexa, they also have great potential for use as a phylogenetic yardstick to determine the evolutionary origin and history of apicomplexan parasites.
Human and Veterinary Medicine
Unlike members of the closely related genus Cyclospora and its more distantly related cousin Cryptosporidium, there is no evidence that any Eimeria species infect humans. In fact, both the nearest relatives to the primates, the Scandentia and the prosimians within the Primates are infected only by Eimeria species, whereas the anthropoid primates, which include the hominids, are only infected by Cystoisospora species, such as Cystoisospora belli, an important human pathogen.
Wild animals other than anthropoids (for example, mice, rabbits, and moles) almost always are infected with 1 or more Eimeria species at one or more times during their life and some might be infected during their entire lives with several species that cycle through them constantly. Given their ubiquitous nature, Eimeria species probably do not often cause discernable pathology or disease under natural conditions, but exceptions exist. For example, E. bovis (Züblin, 1908) Fiebiger, 1912 in cattle, E. tenella (Raillet and Lucet, 1891) Fantham, 1909 in chickens, and E. stiedai (Lindemann, 1865) Kisskalt and Hartmann, 1907 in rabbits all are known to be highly pathogenic in their respective hosts and, recently, another pathogenic species, E. brachylagia Duszynski et al., 2005, was found to cause heavy intestinal infections, some of which resulted in deaths in the endangered Columbia Basin pygmy rabbit, Brachylagus idahoensis, in Washington and Oregon, United States (Duszynski et al., 2005). Eimeria chinchillae has a broad host range and is also highly pathogenic, causing bloody diarrhea, anorexia, severe lesions in the intestines, and ultimately leads to the death of infected animals. It may also cause neurological symptoms (De Vos and Westhuizen, 1968; De Vos, 1970).
When animals are concentrated together, enhancing transmission of Eimeria via its rapid, direct life cycle, some species will cause a disease condition, coccidiosis. Coccidiosis is recognized as a major health hazard: During intensive husbandry of domestic animals; in wild, captive animals such as those in breeding and research facilities and zoos; in wild animal populations when habitat is lost and crowding occurs; and in wild animal species that have great reproductive potential and are protected by laws so that their populations increase inordinately (for example, kangaroos in Australia). All of these conditions are the result of human intervention or perturbation.
Coccidiosis is a serious problem in the poultry industry. In the United States alone, more than 4 trillion birds are raised annually and the United States Department of Agriculture estimated that loss to poultry farmers in the mid-1980s exceeded US$ 80 million when deaths, medicated feeds, and all added labor costs were considered. Worldwide expenditures, just for coccidiostats added to broiler feed, are estimated to be US$ 250–300 million annually. Once a flock becomes infected, especially with 1 or several of the more pathogenic species, a large percentage of the flock can die rapidly. Birds not killed outright by their infection become listless and are more susceptible to predators and other diseases. Even if they survive their infection, they have reduced feed efficiencies. In addition, Eimeria species are becoming widely resistant to the coccidiostats in feed. Similar morbidity and/or mortality and related events occur in cattle feedlots or wherever meat animals are congregated in large numbers.
Coccidiosis is also well documented in some wild species. Eimeria gruis Yakimoff and Matschoulsky, 1935 and E. reichenowi Yakimoff and Matschoulsky, 1935, for example, are common parasites of both whooping cranes and Sandhill cranes in North America and have been reported in other crane species in captivity. These species are considered an important cause of mortality in captive cranes and, during migrations when large numbers of several species of cranes congregate for lengthy periods at watering holes, can be responsible for illness and death in wild populations. Although the disease is generally limited to the intestinal tract in most animals, Eimeria infection in cranes may result in disseminated visceral coccidiosis, where endogenous stages from the gastrointestinal tract become disseminated throughout the body, via the blood or lymphatic systems. Nodules with meront and gamont stages are found in many organs, including lungs, air sacs, trachea, and nares. This disseminated visceral coccidiosis has caused the death of a number of captive Sandhill cranes and whooping cranes. Of the few Eimeria species known to have extraintestinal developmental stages, only the species in cranes can complete their life cycle in both the digestive and respiratory tracts (Carpenter, 1993).
Habitat Destruction, Coccidian Transmission, and Disease
Finally, as the human population continues to grow and agricultural development accelerates to try keep pace, natural places and their endemic faunas will decrease dramatically. The immediate effect of shrinking ecosystems (for example, tropical rain forests, coastal estuaries/wetlands, temperate old-growth forests) is to concentrate both species and individuals into restricted, fragmented areas promoting increased transmission and exchange of parasites, especially those with direct life cycles with resistant oocysts, like many coccidians. Such close contact between host species and their parasites could allow these organisms to become agents of extinction as the host range(s) contract.
Fragmentation increases the edge-effect and can bring an influx of new host species into disturbed or agricultural habitats between fragments, introducing new coccidians and possibly leading to the development of new and more pathogenic strains. Changes in parasite species, intensities, or pathogenicity can have repercussions on the whole food web. The potential, either for domestic animals to become infected by coccidian parasites maintained in wild reservoir host populations, or the reverse, is a strong possibility. For example, we know that deer, elk, or bison can serve as reservoirs for Eimeria and other parasites for domestic livestock and wild rabbits can serve as reservoirs of Eimeria species capable of infecting domesticated rabbits. As humans breed themselves to the brink of extinction and habitat disappears globally at an ever-alarming rate, the potential for biological disaster from the exchange of ubiquitous protozoan parasites, like Eimeria species and its close relatives, may destabilize food webs. Environmental stressors (for example, PCBs), which may compromise host immune systems, global climate change, which challenges the adaptability of host organisms, and the invasion of new parasites, from edge-dwelling hosts, all increase the potential for many apicomplexan parasites to become pathogenic; thus, the importance of disease should be expected to increase in shrinking ecosystems as a consequence of habitat destruction.
Chapter Reviewer
Jana Kvičerová, Department of Parasitology, University of South Bohemia, České Budějovice, Czech Republic.
Literature Cited
Adl, S. M., A. G. B. Simpson, C. E. Lane, J. Lukeš, et al. 2012. The revised classification of Eukaryotes. Journal of Eukaryotic Microbiology 59: 429–493. doi: 10.1111/j.1550-7408.2012.00644.x
Asmundsson, I. M., D. W. Duszynski, and J. A. Campbell. 2006. Seven new species of Eimeria Schneider, 1875 (Apicomplexa: Eimeriidae) from colubrid snakes of Guatemala and a discussion of what to call ellipsoid tetrasporocystic, dizoic coccidian of reptiles. Systematic Parasitology 64: 91–103. doi: 10.1007/s11230-005-9022-6
Baneth, G., M. Samish, and V. Shkap. 2007. Life cycle of Hepatozoon canis (Apicomplexa: Adeleorina: Hepatozoidae) in the tick Rhipicephalus sanguineus and domestic dog (Canis familiaris). Journal of Parasitology 93: 283–299. doi: 10.1645/GE-494R.1
Barta, J. R. 2000. Suborder Adeleorina Léger, 1911. In J. J. Lee, G. F. Leedale, and P. Bradbury, eds. An Illustrated Guide to the Protozoa, Volume 1, 2nd edition. Society of Protozoologists, Lawrence, Kansas, United States, p. 305–318.
Barta, J. R., and R. A. Thompson. 2006. What is Cryptosporidium? Reappraising its biology and phylogenetic affinities. Trends in Parasitology 22: 463–468. doi: 10.1016/j.pt.2006.08.001
Benajiba, M. H., A. Marques, J. Lom, and G. Bouix. 1994. Ultrastructure and sporogony of Eimeria (syn. Epieimeria) anguillae (Apicomplexa) in the eel (Anguilla anguilla). Journal of Eukaryotic Microbiology 41: 215–222. doi: 10.1111/j.1550-7408.1994.tb01500.x
Besnoit, C., and V. Robin. 1912. Sarcosporidiose cutanée chez une vache [= Cutaneous sarcosporidiosis in a cow]. Revue vétérinaire 37: 649–663.
Bjerkås, I., S. F. Mohn, and J. Presthus. 1984. Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Zeitschrift für Parasitenkdunde 70: 271–274.
Blanchard, R. 1885. Note sur les Sarcosporidies et sur un Essai de Classification de ces Sporozoaires [= Note on the Sarcosporidia and on a classification test of these Sporozoa]. Bulletin de la Société zoologique de France 10: 244–276.
Borowski, H., P. L. Clode, and R. C. A. Thompson. 2008. Active invasion and/or encapsulation? A reappraisal of host-cell parasitism by Cryptosporidium. Trends in Parasitology 24: 509–516. doi: 10.1016/j.pt.2008.08.002
Borowski, H., R. C. A. Thompson, T. Armstrong, and P. L. Clode. 2010. Morphological characterization of Cryptosporidium parvum life-cycle stages in an in vitro model system. Parasitology 137: 13–26. doi: 10.1017/S0031182009990837
Bovee, E. C., and S. R. Telford, Jr. 1965. Eimeria sceloporis and Eimeria molochis spp. n. from lizards. Journal of Parasitology 51: 85–94. doi: 10.2307/3275653
Bull, S., R. Chalmers, A. P. Sturdee, A. Curry, et al. 1998. Cross-reaction of an anti-Cryptosporidium monoclonal antibody with sporocysts of Monocystis species. Veterinary Parasitology 77: 195–197. doi: 10.1016/S0304-4017(97)00090-3
Canning, E. U. 1963. The use of histochemistry in the study of sexuality in the coccidia with particular reference to the Adeleidae. In J. Ludvik, J. Lom, J. Vavra, and O. Jirovec, eds. Progress in Protozoology. Academic Press, New York, New York, United States, p. 439–442.
Cardoso, L., H. C. E. Cortes, O. Eyal, A. Reis, et al. 2014. Molecular and histopathological detection of Hepatozoon canis in red foxes (Vulpes vulpes) from Portugal. Parasites and Vectors 7: 113. doi: 10.1186/1756-3305-7-113
Carpenter, J. W. 1993. Infections and parasitic diseases of cranes. In M. E. Fowler, ed. Zoo and Wild Animal Medicine: Current Therapy, Number 3. Saunders, Philadelphia, Pennsylvania, United States, p. 229–237.
Carreno, R. A., D. S. Martin, and J. R. Barta. 1999. Cryptosporidium is more closely related to the gregarines than to coccidia as shown by phylogenetic analysis of apicomplexan parasites inferred using small-subunit ribosomal RNA gene sequences. Parasitology Research 85: 899–904. doi: 10.1007/s004360050
Cavalier-Smith, T. 2014. Gregarine site-heterogeneous 18S rDNA trees, revision of gregarine higher classification, and the evolutionary diversification of Sporozoa. European Journal of Protistology 50: 472–495. doi: 10.1016/j.ejop.2014.07.002
Charles, R. A., A. E. Ellis, J. P. Dubey, J. C. Barnes, et al. 2011. Besnoitiosis in a southern Plains woodrat (Neotoma micropus) from Uvalde, Texas. Journal of Parasitology 97: 838–841. doi: 10.1645/GE-2786.1
Chinchilla, M., and A. Ruiz. 1976. Cockroaches as possible transport hosts of Toxoplasma gondii in Costa Rica. Journal of Parasitology 62: 140–142. doi: 10.2307/3279075
Clowes, C., C. Taylor, J. Folmer, M. Haaramo, et al., 2006. Eukarya: Glossary A–B. Palaeos: Life through Deep Time. http://palaeos.com/eukarya/glossary/glossary.html
Clubb, S. L., and J. K. Frenkel. 1992. Sarcocystis falcatula of opossums: Transmission by cockroaches with fatal pulmonary disease in psittacine birds. Journal of Parasitology 78: 116–124. doi: 10.2307/3283697
Conceição-Silva, F. M., P. Abranches, M. C. D. Silva-Pereira, and J. G. Janz. 1988. Hepatozoonosis in foxes from Portugal. Journal of Wildlife Diseases 24: 344–347. doi: 10.7589/0090-3558-24.2.344
Cornelissen, A. W. C. A., and J. P. Overdulve. 1985. Sex determination and sex differentiation in coccidia: Gametogony and oocyst production after monoclonal infection of cats with free-living and intermediate host stages of Isospora (Toxoplasma) gondii. Parasitology 90: 35–44. doi: 10.1017/S003118200004899X
Cornelissen, A. W. C. A., J. P. Overdulve, and M. Van Der Ploeg. 1984. Determination of nuclear DNA of five Eucoccidian parasites, Isospora (Toxoplasma) gondii, Sarcocystis cruzi, Eimeria tenella, E. acervulina, and Plasmodium berghei, with special reference to gamontogenesis and meiosis in I. (T.) gondii. Parasitology 88: 531–553. doi: 10.1017/S0031182000054792
Craig, T. M. 1990. Hepatozoonosis. In C. E. Greene, ed. Infectious Diseases of the Dog and Cat. Saunders, Philadelphia, Pennsylvania, United States, p. 778–785.
Craig, T. M. 2001. Hepatozoon spp. and hepatozoonosis. In W. M. Samuel, M. J. Pybus, and A. A. Kocan, eds. Parasitic Diseases of Wild Mammals. Iowa State University Press, Ames, Iowa, United States, p. 462–468.
Darling, S. T. 1910. Sarcosporidiosis in an opossum and its experimental production in the guinea pig by the intramuscular injection of sporozoites. Bulletin de la Société de pathologie exotique 3: 513–518.
De Vos, A. J. 1970. Studies on the host range of Eimeria chinchillae De Vos and Van der Westhuizen, 1968. Ondersteeport Journal of Veterinary Research 37: 29–36.
De Vos, A. J., and I. B. van der Westhuizen. 1968. The occurrence of Eimeria chinchillae n. sp. (Eimeriidae) in Chinchilla laniger (Molina, 1782) in South Africa. Journal of the South African Veterinary Medical Association 39: 81–82.
Dubey, J. P. 1975. Experimental Isospora canis and Isospora felis infection in mice, cats, and dogs. Journal of Protozoology 22: 416–417. doi: 10.1111/j.1550-7408.1975.tb05195.x
Dubey, J. P. 1978a. Life cycle of Isospora ohioensis in dogs. Parasitology 77: 1–11.
Dubey, J. P. 1978b. Pathogenicity of Isospora ohioensis infection in dogs. Journal of the American Veterinary Medical Association 173: 192–197.
Dubey, J. P. 1999. Recent advances in Neospora and neosporosis. Veterinary Parasitology 84: 349–367. doi: 10.1016/S0304-4017(99)00044-8
Dubey, J. P. 1976. A review of Sarcocystis of domestic animals and of other coccidia of cats and dogs. Journal of the American Veterinary Medical Association 169: 1,061–1,078.
Dubey, J. P. 2010. Toxoplasma gondii infections in chickens (Gallus domesticus): Prevalence, clinical disease, diagnosis, and public health significance. Zoonoses and Public Health 57: 60–73. doi: 10.1111/j.1863-2378.2009.01274.x
Dubey, J. P. 1998. Toxoplasma gondii oocyst survival under defined temperatures. Journal of Parasitology 84: 862–865. doi: 10.2307/3284606
Dubey, J. P., and C. P. Beattie. 1988. Toxoplasmosis of Animals and Man. CRC Press, Boca Raton, Florida, United States, 220 p.
Dubey, J. P., and J. K. Frenkel. 1972. Cyst-induced toxoplasmosis in cats. Journal of Protozoology 19: 155–177. doi: 10.1111/j.1550-7408.1972.tb03431.x
Dubey, J. P., R. Calero-Bernal, B. M. Rosenthal, C. A. Speer, et al. 2015. Sarcocystis of Animals and Humans, 2nd edition. CRC Press, Boca Raton, Florida, United States, 501 p.
Dubey, J. P., J. L. Carpenter, C. A. Speer, M. J. Topper, et al. 1988. Newly recognized fatal protozoan disease of dogs. Journal of the American Veterinary Medical Association 192: 1,269–1,285.
Dubey, J. P., A. Hemphill, R. Calero-Bernal, and G. Schares. 2017. Neosporosis in Animals. CRC Press, Boca Raton, Florida, United States, 448 p.
Dubey, J. P., C. Sreekumar, D. S. Lindsay, D. Hill, et al. 2003. Besnoitia oryctofelis n. sp. (Protozoa: Apicomplexa) from domestic rabbits. Parasitology 126: 521–539. doi: 10.1017/S0031182003003123
Duszynski, D. W. 2016. The Biology and Identification of the Coccidia (Apicomplexa) of Marsupials of the World. Elsevier/Academic Press, London, United Kingdom, 241 p.
Duszynski, D. W. 2002. Coccidia (Apicomplexa: Eimeriidae) of the mammalian order Chiroptera. Special Publication of the Museum of Southwestern Biology, Number 5. University of New Mexico Printing Services, Albuquerque, New Mexico, United States, 45 p.
Duszynski, D. W., and L. Couch. 2013. The Biology and Identification of the Coccidia (Apicomplexa) of Rabbits of the World. Elsevier/Academic Press, London, United Kingdom, 340 p.
Duszynski, D. W., and J. J. Morrow. 2014. The Biology and Identification of the Coccidia (Apicomplexa) of Turtles of the World. Elsevier/Academic Press, London, United Kingdom, 210 p.
Duszynski, D. W., and S. J. Upton. 2010. The Biology of the Coccidia (Apicomplexa) of Snakes of the World: A Scholarly Handbook for Identification and Treatment. CreateSpace, Scotts Valley, California, United States, 422 p.
Duszynski, D. W., and S. J. Upton. 2000. Coccidia (Apicomplexa: Eimeriidae) of the mammalian order Insectivora. Special Publications of the Museum of Southwestern Biology, Number 4. University of New Mexico, Albuquerque, New Mexico, United States, 67 p.
Duszynski, D. W., and S. J. Upton. 2001. Cyclospora, Eimeria, Isospora, and Cryptosporidium spp. In W. M. Samuel, M. J. Pybus, and A. A. Kocan, eds. Parasitic Diseases of Wild Mammals, 2nd edition. Iowa State University Press, Ames, Iowa, United States, p. 416–459.
Duszynski, D. W., and P. G. Wilber. 1997. A guideline for the preparation of species descriptions in the Eimeriidae. Journal of Parasitology 83: 333–336. doi: 10.2307/3284470
Duszynski, D. W., M. G. Bolek, and S. J. Upton. 2007. Coccidia (Apicomplexa: Eimeriidae) of amphibians of the world. Zootaxa 1667: 1–77. doi: 10.11646/zootaxa.1667.1.1
Duszynski, D. W., L. Harrenstien, L. Couch, and M. M. Garner. 2005. A pathogenic new species of Eimeria from the pygmy rabbit, Brachylagus idahoensis, in Washington and Oregon, with description of the sporulated oocyst and intestinal endogenous stages. Journal of Parasitology 91: 618–623. doi: 10.1645/GE-435R
Duszynski, D. W., C. T. McAllister, and M. Tellez. 2020. The coccidia (Apicomplexa) of the Archosauria (Crocodylia: Eusuchia) of the world. Journal of Parasitology 106: 90–122. doi: 10.1645/19-73
Duszynski, D. W., W. D. Wilson, S. J. Upton, and N. D. Levine. 1999. Coccidia (Apicomplexa: Eimeriidae) in the Primates and Scandentia. International Journal of Primatology 20: 761–797. doi: 10.1023/A:102070892
Dyková, I., and J. Lom. 1981. Fish coccidia: Critical notes on life cycles, classification and pathogenicity. Journal of Fish Diseases 4: 487–505. doi: 10.1111/j.1365-2761.1981.tb01161.x
Edgcomb, J. H., D. H. Walker, and C. M. Johnson. 1976. Klossiella in the opossum. Veterinary Pathology 13: 315–318. doi: 10.1177/030098587601300408
Fayer, R. 1972. Gametogony of Sarcocystis sp. in cell culture. Science 175: 65–67. doi: 10.1126/science.175.4017.65
Fayer, R. 1970. Sarcocystis: Development in cultured avian and mammalian cells. Science 168: 1,104–1,105. doi: 10.1126/science.168.3935.1104
Fayer, R. 2010. Taxonomy and species delimitation in Cryptosporidium. Experimental Parasitology 124: 90–97. doi: 10.1126/science.175.4017.65
Fayer, R., and J. P. Dubey. 1987. Comparative epidemiology of coccidia: Clues to the etiology of equine protozoal myeloencephalitis. International Journal for Parasitology 17: 615–620. doi: 10.1016/0020-7519(87)90138-X
Fayer, R., U. Morgan, and S. J. Upton. 2000. Epidemiology of Cryptosporidium transmission, detection and identification. International Journal for Parasitology 30: 1,305–1,322. doi: 10.1016/S0020-7519(00)00135-1
Fayer, R., M. Santín, and D. Macarisin. 2010. Cryptosporidium ubiquitum n. sp. in animals and humans. Veterinary Parasitology 172: 23–32. doi: 10.1016/j.vetpar.2010.04.028
Fayer, R., M. Santín, J. M. Trout, and J. P. Dubey. 2006. Detection of Cryptosporidium felis and Giardia duodenalis Assemblage F in a cat colony. Veterinary Parasitology 140: 44–53. doi: 10.1016/j.vetpar.2006.03.005
Feng, Y., T. Dearen, V. Cama, and L. Xiao. 2009. 90-kilodalton heat shock protein, Hsp90, as a target for genotyping Cryptosporidium spp. known to infect humans. Eukaryotic Cell 8: 478–482. doi: 10.1128/EC.00294-08
Frenkel, J. K. 1977. Besnoitia wallacei of cats and rodents: With a reclassification of other cyst-forming isosporoid coccidia. Journal of Parasitology 63: 611–628. doi: 10.2307/3279560
Frenkel, J. K., and J. P. Dubey. 1973. Effects of freezing on the viability of Toxoplasma oocysts. Journal of Parasitology 59: 587–588. doi: 10.2307/3278803
Frenkel, J. K., and J. P. Dubey. 1972. Rodents as vectors for feline coccidia, Isospora felis and Isospora rivolta. Journal of Infectious Diseases 125: 69–72.
Frenkel, J. K., H. Mehlhorn, and A. O. Heydorn. 1987. Beyond the oocyst: Over the molehills and mountains of coccidialand [Letters]. Parasitology Today 3: 250–252. doi: 10.1016/0169-4758(87)90151-7
Frenkel, J. K., A. Ruiz, A., and M. Chinchilla. 1975. Soil survival of Toxoplasma oocysts in Kansas and Costa Rica. American Journal of Tropical Medicine and Hygiene 24: 439–443. doi: 10.4269/ajtmh.1975.24.439
Furtado, M. M., B. Metzger, A. T. de Almeida Jácomo, M. B. Labruna, et al. 2017. Hepatozon spp. infect free-ranging jaguars (Panthera onca) in Brazil. Journal of Parasitology 103: 243–250. doi: 10.1645/16-99
Gao, S. S., S. Q. Wu, J. Luo, C. M. Wang, et al. 2013. [Development of an IMS-qPCR method for detection of Cryptosporidium parvum in water.] Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi [= Chinese Journal of Parasitology and Parasitic Diseases] 31: 180–184. [In Chinese.]
Gardner, M. J., N. Hall, E. Fung, O. White, et al. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419: 498–511. doi: 10.1038/nature01097
Ghimire, T. R., 2010. Redescription of genera of family Eimeriidae Minchin, 1903. International Journal of Life Sciences 4: 26–47. doi: 10.3126/ijls.v4i0.3285
Goodwin, M. A., and W. D. Waltman. 1996. Transmission of Eimeria, viruses, and bacteria to chicks: Darkling beetles (Alphitobius diaperimus) as vectors of pathogens. Journal of Applied Poultry Research 5: 51–55. doi: 10.1093/japr/5.1.51
Haberkorn, A. 1970. Die Entwicklung von Eimeria falciformis (Eimer 1870) in der weissen Maus (Mus musculus). Zeitschrift für Parasitenkunde 34: 49–67.
Henry, A. 1913. Le travail de M. M. Besnoit et Robin [= The work of M. M. Besnoit and Robin]. Également communique a la Société des sciences vétérinaires de Lyon (Séance du 17 Novembre 1912). Revue médicine vétérinaire 90: 328.
Hijjawi, N. S., B. P. Meloni, U. M. Ryan, and M. E. Olson, et al. 2002. Successful in vitro cultivation of Cryptosporidium andersoni: Evidence for the existence of novel extracellular stages in the life cycle and implications for the classification of Cryptosporidium. International Journal for Parasitology 32: 1,719–1,726.
Hnida, J. A., and D. W. Duszynski. 1999. Cross-transmission studies with Eimeria arizonensis, E. arizonensis-like oocysts and E. langebarteli: Host specificity within the Muridae and other rodents. Journal of Parasitology 85: 873–877. doi: 10.2307/3285824
Homem, C. G., A. A. Nakamura, D. C. Silva, W. F. Teixeira, et al. 2012. Real-time PCR assay targeting the actin gene for the detection of Cryptosporidium parvum in calf fecal samples. Parasitology Research 110: 1,741–1,745. doi: 10.1007/s00436-011-2694-8
Houk, A. E., A. C. Rosypal, D. C. Grant, J. P. Dubey, et al. 2011. Serological response of cats to experimental Besnoitia darlingi and Besnoitia heotomofelis infections and prevalence of antibodies to these parasites in cats from Virginia and Pennsylvania. Journal of Parasitology 97: 259–261. doi: 10.1645/GE-2626.1
Huet, L. 1882. Note sur des parasites trouves dans les poumons et dans les muscles de l’Otaria californiana [= Note on parasite found in the lungs and muscles of Otaria californiana]. Comptes rendus des Memórias seances Société de biologie 34: 321–322.
Ivanov, A., and I. Tsachev. 2008. Hepatozoon canis and hepatozoonosis in the dog. Trakia Journal of Sciences 6: 27–35.
Jeffers, T. K. 1978. Genetics of coccidia and the host response. In P. L. Long, K. N. Boorman, and B. M. Freeman, eds. Avian Coccidiosis. British Poultry Science, Edinburgh, United Kingdom, p. 51–125.
Jellison, W. L. 1956. On the nomenclature of Besnoitia besnoiti, a protozoan parasite. Annals of the New York Academy of Sciences 64: 268–270. doi: 10.1111/j.1749-6632.1956.tb36618.x
Jiang, J., and L. Xiao. 2003. An evaluation of molecular diagnostic tools for the detection and differentiation of human‐pathogenic Cryptosporidium spp. Journal of Eukaryotic Microbiology 50 (Supplement): 542–547. doi: 10.1111/j.1550-7408.2003.tb00623.x
Jirků, M., M. Jirků, M. Oborník, J. Lukeš, et al. 2009. A model for taxonomic work on homoxenous Coccidia: Redescription, host specificity, and molecular phylogeny of Eimeria ranae Dobell, 1909, with a review of anuran‐host Eimeria (Apicomplexa: Eimeriorina). Journal of Eukaryotic Microbiology 56: 39–51. doi: 10.1111/j.1550-7408.2008.00362.x
Klimes, B., D. G. Rootes, and Z. Tanielian. 1972. Sexual differentiation of merozoites of Eimeria tenella. Parasitology 65: 131–136. doi: 10.1017/S0031182000044292
Koh, W., R. C. A. Thompson, H. Edwards, P. Monis, et al. 2014. Extracellular excystation and development of Cryptopsoridium: Tracing the fate of oocysts within Pseudomonas aquatic biofilm systems. BMC Microbiology 14: 281.
Kuo, C. H., J. P. Wares, and J. C. Kissinger. 2008. The apicomplexan whole-genome phylogeny: An analysis of incongruence among gene trees. Molecular Biology and Evolution 25: 2,689–2,698. doi: 10.1093/molbev/msn213
Labbé, A. 1899. Sporozoa. In F. E. Schulze and O. Butschi, eds. Tierreich. Friedlander, Berlin, Germany, p. 115–119.
Lainson, R., and I. Paperna. 1999. Some coccidia from the gall-bladder and intestine of the teiid lizard Ameiva ameiva ameiva and the gecko Hemidactylus mabouia in North Brazil. Parasite 6: 151–162. doi: 10.1051/parasite/1999062151
Lalonde, L.F., J. Reyes, and A. A. Gajadhar. 2013. Application of a qPCR assay with melting curve analysis for detection and differentiation of protozoan oocysts in human fecal samples from Dominican Republic. American Journal of Tropical Medicine and Hygiene 89: 892–898.
Lankester, E. R. 1882. On Drepanidium ranarum the cell parasite of the frog’s blood and spleen (Gaule’s Wurmchen). Quarterly Journal of Microscopy 12: 53–65. doi: 10.4269/ajtmh.13-0106
Lee, E.-H., O. Remmler, and M. A. Fernando. 1977. Sexual differentiation in Eimeria tenella (Sporozoa: Coccidia). Journal of Parasitology 63: 155–156. doi: 10.2307/3280127
Leighton, F. A., and A. A. Gajadhar. 2001. Tissue inhabiting protozoans. In W. M. Samuel, M. J. Pybus, and A. A. Kocan, eds. Parasitic Diseases of Wild Mammals. Iowa State University Press, Ames, Iowa, United States, p. 468–478.
Leoni, F., C. I. Gallimore, J. Green, and J. McLauchlin. 2006. Characterisation of small double stranded RNA molecule in Cryptosporidium hominis, Cryptosporidium felis and Cryptosporidium meleagridis. Parasitology International 55: 299–306. doi: 10.1016/j.parint.2006.06.006
Levine, N. D. 1973. Historical aspects of research on coccidiosis. In Proceedings of the Symposium on Coccidia and Related Organisms. University of Guelph, Guelph, Ontario, Canada, p. 1–10.
Levine, N. D. 1940. The initiation of avian coccidial infection with merozoites. Journal of Parasitology 26: 337–343. doi: 10.2307/3272478
Levine, N. D., and V. Ivens. 1965. Isospora species in the dog. Journal of Parasitology 51: 859–864. doi: 10.2307/3276177
Lindergard, G., D. V. Nydam, S. E. Wade, S. L. Schaaf, et al. 2003. A novel multiplex polymerase chain reaction approach for detection of four human infective Cryptosporidium isolates: Cryptosporidium parvum, types H and C, Cryptosporidium canis, and Cryptosporidium felis in fecal and soil samples. Diagnostic Investigation 15: 262–267. doi: 10.1177/104063870301500307
Lindsay, D. S., and J. P. Dubey. 2000. Canine neosporosis. Journal of Veterinary Parasitology 14: 1–11.
Lindsay, D. S., and K. S. Todd, Jr. 1993. Coccidia of mammals. In Parasitic Protozoa, Volume 4. Academic Press, New York, New York, United States, p. 89–131.
Lucio-Forster, A., J. K. Griffiths, V. A. Cama, L. Xiao, et al. 2010. Minimal zoonotic risk of cryptosporidiosis from pet dogs and cats. Trends in Parasitology 26: 174–179. doi: 10.1016/j.pt.2010.01.004
Markus, M. B. 1974. Earthworms and coccidian oocysts. Annals of Tropical Medicine and Parasitology 68: 247–248. doi: 10.1080/00034983.1974.11686947
Markus, M. B. 1980. Flies as natural transport hosts of Sarcocystis and other coccidia. Journal of Parasitology 66: 361–362. doi: 10.2307/3280842
Marotel, M. 1912. Discussion paper by Besnoit and Robin. Bulletin et Mémoire de la Société des sciences vétérinaires de Lyon et de la Société de médecine vétérinaire des Lyon et du Sud-Est 15: 196–217.
Matsui, T., T. Morii, T. Iijima, F. Kobayashi, et al. 1989. Transformation of oocysts from several coccidian species by heat treatment. Parasitology Research 75: 264–267. doi: 10.1007/BF00931810
McAllister, M. M., J. P. Dubey, D. S. Lindsay, W. R. Jolley, et al. 1998. Dogs are definitive hosts of Neospora caninum. International Journal for Parasitology 28: 1,473–1,478. doi: 10.1016/S0020-7519(98)00138-6
McGlade, T. R., E. D. Robertson, A. D. Elliot, C. Read, et al. 2003. Gastrointestinal parasites of domestic cats in Perth, Western Australia. Veterinary Parasitology 117: 251–262. doi: 10.1016/j.vetpar.2003.08.010
Meireles, M. V. 2010. Cryptosporidium infection in Brazil: Implications for veterinary medicine and public health. Revista Brasileira de Parasitologia Veterinaria 19: 197–204. doi: 10.1590/S1984-29612010000400002
Merino, S., J. Martínez, R. A. Vasquez, and J. Šlapeta. 2010. Monophyly of marsupial intraerythrocytic apicomplexan parasites from South America and Australia. Parasitology 137: 37–43. doi: 10.1017/S0031182009990710
Merino, S., R. A. Vásquez, J. Martínez, J. L. Celis-Diez, et al. 2009. Molecular characterization of an ancient Hepatozoon species parasitizing the “living fossil” marsupial “Monito del Monte” Dromiciops gliroides from Chile. Biological Journal of the Linnean Society 98: 568–576. doi: 10.1111/j.1095-8312.2009.01302.x
Merino, S., R. A. Vásquez, J. Martínez, J. L. Celis-Diez, et al. 2008. A sarcocystid misidentified as Hepatozoon didelphydis: Molecular data from a parasitic infection in the blood of the southern mouse opossum (Thylamys elegans) from Chile. Journal of Eukaryotic Microbiology 55: 536–540. doi: 10.1111/j.1550-7408.2008.00358.x
Miescher, F. 1843. Über eigenthümliche Schläuche in den Muskein einer Hausmaus [= On peculiar tubes in the muscle of a house mouse]. Bericht der Verhandlungen der Naturforschender Gesellschaft 5: 198–202.
Modrý, D., J. Votýpka, and M. Svobodová. 2004. Note on the taxonomy of Frenkelia microti (Findlay & Middleton 1934) (Apicomplexa, Sarcocystidae). Systematic Parasitology 58: 185–187. doi: 10.1023/B:SYPA.0000032924.63708.57
Morgan, U. M., and R. C. Thompson. 1998. PCR detection of Cryptosporidium: The way forward? Trends in Parasitology 14: 241–245. doi: 10.1016/S0169-4758(98)01247-2
Mottalei, F., L. F. Mayberry, and J. R. Bristol. 1992. Localization of extraintestinal Eimeria nieschulzi (Apicomplexa: Eimeriidae) stages in the rat utilizing an indirect immunofluorescence technique. Transactions of the American Microscopic Society 111: 61–64.
Nowak, R. M. 1991. Walker’s Mammals of the World, Volume 1, 5th edition. Johns Hopkins University Press, Baltimore, Maryland, United States, 642 p.
O’Dwyer, L. H., C. L. Massard, and J. C. P. de Souza. 2001. Hepatozoon canis infection associated with dog ticks of rural areas of Rio de Janeiro State, Brazil. Veterinary Parasitology 94: 143–150. doi: 10.1016/S0304-4017(00)00378-2
Osman, M., J. Bories, D. El-Safadi, M. T. Poirel, et al. 2015. Prevalence and genetic diversity of the intestinal parasites Blastocystis sp. and Cryptosporidium spp. in household dogs in France and evaluation of zoonotic transmission risk. Veterinary Parasitology 214: 167–170. doi: 10.1016/j.vetpar.2015.09.015
Paperna, I., 1991. Fine structure of Eimeria (s. l.) vanasi merogony stages in the intestinal mucosa of cichlid fishes. Diseases of Aquatic Organisms 10: 195–201. doi: 10.3354/dao010195
Paperna, I., and J. H. Landsberg. 1989. Description and taxonomic discussion of eimerian coccidia from African and Levantine geckoes. South African Journal of Zoology 24: 345–355. doi: 10.1080/02541858.1989.11448176
Plutzer, J., and P. Karanis. 2009. Genetic polymorphism in Cryptosporidium species: An update. Veterinary Parasitology 165: 187–199. doi: 10.1016/j.vetpar.2009.07.003
Pratt, H. D., and K. S. Littig. 1962. Ticks of public health importance and their control: Training guide. [Insect control series: Part X. Public Health Service publication number 772.] United States Public Health Service, Communicable Disease Center, Atlanta, Georgia, United States, X-42 p.
Rausch, R. L. 1952. Hydatid disease in boreal regions. Arctic: Journal of the Arctic Institute of North America 5: 157–174.
Rommel, M., and B. Zielasko. 1981. Untersuchungen uber den Lebenszyklus von Isospora burrowsi (Trayser und Todd, 1978) aus dem Hund. Berliner und Münchener Tierärztliche Wochenschrift 94: 87–90.
Rommel, M., A.-O. Heydorn, and F. Gruber. 1972. Beiträge zum Lebenszyklus der Sarkosporidien, I: Die Sporozyte von S. tenella in den Fäzes der Katze. Berliner und Münchener Tierärztliche Wochenschrift 85: 101–105.
Ryan, U., and M. Power. 2012. Cryptosporidium species in Australian wildlife and domestic animals. Parasitology 139: 1,673–1,688. doi: 10.1017/S0031182012001151
Sakuma, M., T. Nishio, N. Nakanishi, M. Izawa, et al. 2011. A case of Iriomote Cat (Prionilurus bengalensis iriomotensis) with Hepatozoon felis parasitemia. Journal of Veterinary Medical Science 73: 1,381–1,384. doi: 10.1292/jvms.11-0210
Sam-Yellowe, T. Y. 1996. Rhoptry organelles of the Apicomplexa: Their role in host cell invasion and intracellular survival. Parasitology Today 12: 308–315. doi: 10.1016/0169-4758(96)10030-2
Scholtyseck, E. 1979. Fine structure of parasitic Protozoa. In An Atlas of Micrographs, Drawings, and Diagrams. Springer Science and Business Media, Berlin, West Germany.
Scorza, A. V., M. M. Brewer, and M. R. Lappin. 2003. Polymerase chain reaction for the detection of Cryptosporidium spp. in cat feces. Journal of Parasitology 89: 423–426. doi: 10.1645/0022-3395(2003)089[0423:PCRFTD]2.0.CO;2
Scorza, J. V., J. F. Torrealba, and C. Dagert. 1957. Klossiella tejerai nov. sp. y Sarcocystis didelphidis nov. sp. parasitos de un Didelphis marsupialis de Venezuela. Acta Biológica Venezuelica 2: 97–108.
Shirley, M. W., and B. J. Millard. 1976. Some observations on the sexual differentiation of Eimeria tenella using single sporozoite infections in chicken embryos. Parasitology 73: 337–341. doi: 10.1017/S0031182000047016
Silva, S. O., L. J. Richtzenhain, I. N. Barros, A. M. Gomes, et al. 2013. A new set of primers directed to 18S rRNA gene for molecular identification of Cryptosporidium spp. and their performance in the detection and differentiation of oocysts shed by synanthropic rodents. Experimental Parasitology 135: 551–557. doi: 10.1016/j.exppara.2013.09.003
Smith, D. D., and J. K. Frenkel. 1978. Cockroaches as vectors of Sarcocystis muris and other coccidia in the laboratory. Journal of Parasitology 64: 315–319. doi: 10.2307/3279682
Smith, T., and H. P. Johnson. 1902. On a coccidium (Klossiella muris, gen. et spec. nov.) parasitic in the renal epithelium of the mouse. Journal of Experimental Medicine 6: 303–316. doi: 10.1084/jem.6.3.303
Spitz dos Santos, C., B. P. Berto, B. Lopes, B. Do, et al. 2014. Coccidial dispersion across New World marsupials: Klossiella tejerai Scorza, Torrealba and Dagert, 1957 (Apicomplexa: Adeleorina) from the Brazilian common opossum Didelphis aurita (Wied-Neuwied) (Mammalia: Didelphimorphia). Systematic Parasitology 89: 83–89. doi: 10.1007/s11230-014-9510-7
Tenter, A. M., J. R. Barta, I. Beveridge, D. W. Duszynski, et al. 2002. The conceptual basis for a new classification of the coccidia. International Journal for Parasitology 32: 595–616.
Thompson, R. C. A., W. H. Koha, and P. L. Clode. 2016. Cryptosporidium: What is it? Food and Water Parasitology 4: 54–61. doi: 10.1016/S0020-7519(02)00021-8
Tyzzer, E. E. 1910. An extracellular coccidium, Cryptosporidium muris (gen. et sp. nov.), of the gastric glands of the common mouse. Journal of Medical Research 23: 487–509.
Tyzzer, E. E. 1907. A sporozoan found in the peptic glands of the common mouse. Proceedings of the Society for Experimental Biology and Medicine 5: 12–13. doi: 10.3181/00379727-5-5
Tzipori, S., and I. Campbell. 1981. Prevalence of Cryptosporidium antibodies in 10 animal species. Journal of Clinical Microbiology 14: 455–456.
Tzipori, S., K. W. Angus, I. Campbell, and E. W. Gray. 1980. Cryptosporidium: Evidence for a single-species genus. Infection and Immunity 30: 884–886.
Uetz, P., P. Freed, and J. Hošek, eds. 2018. The Reptile Database. http://www.reptile-database.org
Valigurová, A., N. Vaskovicová, N. Musilová, and J. Schrével. 2013. The enigma of eugregarine epicytic folds: Where gliding motility originates? Frontiers in Zoology 10: 57. doi: 10.1186/1742-9994-10-57.
Vincent-Johnson, N. A., D. K. Macintire, D. S. Lindsay, S. D. Lenz, et al. 1997. A new Hepatozoon species from dogs: Description of the causative agent of canine hepatozoonosis in North America. Journal of Parasitology 83: 1,165–1,172. doi: 10.2307/3284379
Votýpka, J, V. Hypša, M. Jirků, J. Flegr, et al. 1998. Molecular phylogenetic relatedness of Frenkelia spp. (Protozoa, Apicomplexa) to Sarcocystis falcatula Stiles 1893: Is the genus Sarcocystis paraphyletic? Journal of Eukaryotic Microbiology 45: 137–141. doi: 10.1111/j.1550-7408.1998.
tb05081.x
Wallace, G. D. 1971. Experimental transmission of Toxoplasma gondii by filth-flies. American Journal of Tropical Medicine and Hygiene 20: 411–413. doi: 10.4269/ajtmh.1971.20.411
Weiss, L. M., and K. Kim, eds. 2007. Toxoplasma gondii, the Model Apicomplexan: Perspectives and Methods. Elsevier/Academic Press, London, United Kingdom, 777 p.
Wilber, P. G., D. W. Duszynski, S. J. Upton, R. S. Seville, et al. 1998. A revision of the taxonomy and nomenclature of the eimerians (Apicomplexa: Eimeriidae) from rodents in the tribe Marmotini (Sciuridae). Systematic Parasitology 39: 113–135. doi: 10.1023/A:100591401
Williams, R. B., P. Thebo, R. N. Marshall, and J. A. Marshall. 2010. Coccidian oocysts as type-specimens: Long-term storage in aqueous potassium dichromate solution preserves DNA. Systematic Parasitology 76: 69–76. doi: 10.1007/s11230-010-9234-2
Wilson, D. E., and D. A. M. Reeder, eds. 2005. Mammal Species of the World: A Taxonomic and Geographic Reference, Volumes 1 and 2, 3rd edition. Johns Hopkins University Press, Baltimore, Maryland, United States.
Xiao, L., R. Fayer, U. Ryan, and S. J. Upton. 2004. Cryptosporidium taxonomy: Recent advances and implications for public health. Clinical Microbiology Reviews 17: 72–97. doi: 10.1128/cmr.17.1.72-97.2004
Supplemental Reading
Adl, S. M., A. G. B. Simpson, M. A. Farmer, R. A. Andersen, et al. 2005. The new higher-level classification of eukaryotes with emphasis on the taxonomy of protists. Journal of Eukaryotic Microbiology 52: 399–451. doi: 10.1111/j.1550-7408.2005.00053.x
Dubey, J. P. 1975. Isospora ohioensis sp. n. proposed for I. rivolta of the dog. Journal of Parasitology 61: 462–465. doi: 10.2307/3279325
Duszynski, D. W. 2021. Biodiversity of the Coccidia (Apicomplexa: Conoidasida) in vertebrates: What we know, what we do not know, and what needs to be done. Folia Parasitologica 68: 2021.001. doi: 10.14411/fp.2021.001
Duszynski, D. W., J. Kvičerová, J., and R. S. Seville. 2018. The Biology and Identification of the Coccidia (Apicomplexa) of Carnivores of the World. Elsevier/Academic Press, London, United Kingdom, 712 p.
Noun
Plural: syzygies
From Greek: syzygos = united
Definition 1: The combining of organs without loss of identity
Definition 2: Among the Crinoidea, having each nodal columnal closely and rigidly jointed to the internodal columnal below it by short elastic fibers, and as such lacking flexibility
Noun
From Greek: gamete = wife
Definition: A cell that unites with another cell in sexual reproduction
Adjective
From Greek: homo = same; xenos = guest/host/stranger
Definition: Having just one host during a parasite's life cycle
Adjective
From Greek: heteros = different; xenos = host/guest/stranger
Definition: Having more than one host during a parasite's life cycle
Noun
From Greek: spora = seed; gonos = offspring
Definition: The multiple fission of a zygote; a sporont
Definition: Host in which the terminal (frequently sexual) stage of the parasite occurs
Synonym: Primary host
Noun
From Latin: vehere = to carry
Definition 1: Any carrier, particularly an animal, that transmits a disease organism from one host to another
Defintion 2: In helminthic disease, an intermediate host that seeks out the definitive host; such as a mosquito
Noun
From Greek: oion = egg; kystis =pouch
Definition: The cystic form in the parasitic protozoans (Apicomplexa), resulting from sporogony; may be hard covered with a resistant membrane (as in Eimeria) or be naked (as in Plasmodium)
Noun
From Greek: spora = seed; kystis = bladder
Definition 1: A stage of sporozoan development, usually within a protective envelope; the oocyst
Definition 2:. Among trematodes, an asexual stage of development
Noun
From Greek: spora = seed; zoon = animal
Definition: The stage of development of a sporoblast which has divided and exited the oocyst into the hemocoel and migration begins; the malarial stage found in the salivary glands of insects
Noun
From Greek: haima = blood; koilos = hollow
Definition 1: Among arthropods, the main body cavity, the embryonic development of which differs from that of a true coelom, but which includes a vestige of that true coelom that emanates from the blood spaces of the embryo, or remnants of the blastocoel after invasion of the latter by the mesoderm
Definition 2: Among molluscs, the main body cavity
Adjective
From Greek: para = beside; phyletes = tribesman
Definition: A monophyletic group that does not contain all of the descendants of the most recent common ancestor of that group
Noun
From Greek: gamete = wife; kytos = container
Definition 1: A spermatocyte or oocyte
Definition 2: Sexual stage of the malarial parasite in the blood which upon being taken into the mosquito host may produce gametes
Noun
From Greek: kytos = container; plasma = formed or molded
Definition: The protoplasm of a cell excluding the nucleus, usually a slightly viscous fluid with inclusions suspended in it; the site of the chemical activities of the cell

