Refer to the tables below the question as you answer the question.
| Resource | Monographs | Custom Reports | Publisher/Price |
|---|---|---|---|
| DailyMed | *FDA-approved Package Inserts (labels)
*No off-label uses *Manufacturer-specific inactive ingredients are listed |
None | The DailyMed interface is a US government product and is freely available.
The individual package inserts are published by the manufacturers and just made available by DailyMed
|
| Interface/Resource or part of interface | Monographs | Custom Reports | Publisher/Price |
|---|---|---|---|
| Clinical Pharmacology/Tools | ...................... | *Consumer-level interactions report
*Professional-level Adverse reactions report Professional-level interactions report Profesional-level IV compatibility report (based on Trissel's) |
Elsevier/$$ |
| Clinical Pharmacology/Monographs | standard info | ......................... | Elsevier/$$ |
| UpToDate LexiDrug/Tools | .......................... | Professional-level interactions report
Profesional-level IV compatibility report (based on Trissel's) |
Wolters Kluwer/$$ |
| UpToDate LexiDrug/LexiDrugs | *includes drug prices | ............................. | Wolters Kluwer/$$ |
| UpToDate LexiDrug/Martindale: The Complete Drug Reference | *includes English and foreign generic names, scientific names, US and foreign brand/trade names, and CAS registry numbers | ............................... | Wolters Kluwer/$$ |
| UpToDate LexiDrug/AHFS DI | *pearls of information not found elsewhere | ............................... | American Society of Health-System Pharmacists, Inc./$$ |
To remember that Clinical Pharmacology provides custom, adverse reaction reports, think of this:

Return to Clinical Pharmacology:
- Click here to return to Clinical Pharmacology and pull the new window/tab off of the instruction panel and onto your working window.
To create the "Adverse Reaction" report that you need:
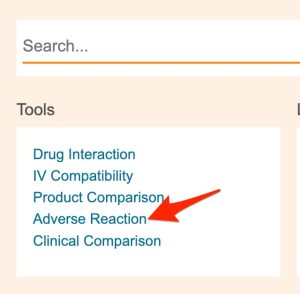
- Use Clinical Pharmacology's "Tools" menu to select the "Adverse Reaction" report tool.
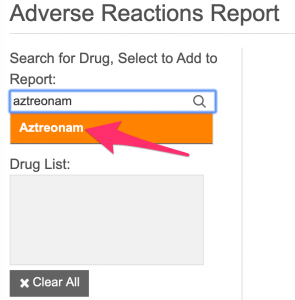
- Enter Mrs. Dilworth's medications (one-by-one) into the "Add drug..." box. Mrs. Dilworth's medications include:
aztreonam
clindamycin
risperidone
ondansetron
- After entering a drug's name, hit the keyboard "enter" key or click the name of the appropriate drug in the list that appears below the search box (see red arrow in figure below).
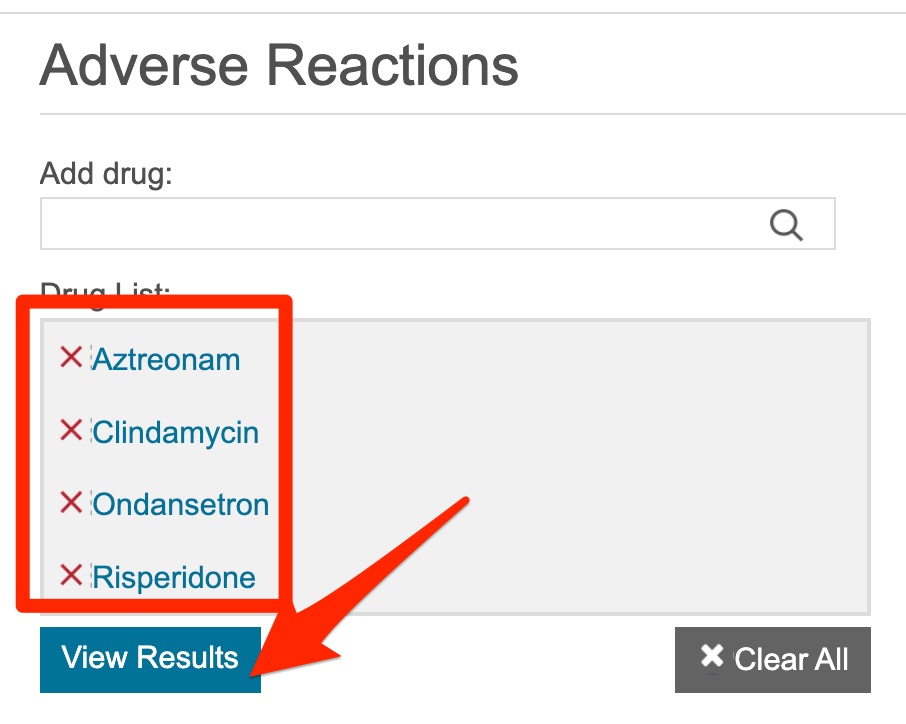
- After you have added all the drug names to the "Drug list", click on the circle in front of "By frequency"
- Click the "View Results" button.
- When the report appears, find the entry for --
pruritus
While three of the drugs the patient is receiving might cause the itching, the fact that the itching started soon after initiation of the ondansetron makes you suspect that the ondansetron is responsible in this patient. Also, the fact that Mrs. Dilworth has been taking risperidone for a long time makes this medication a less likely culprit.
One of the team members asks whether systemic clindamycin causes itching or whether this adverse effect is only seen after topical administration.
- Click on the "clindamycin" link next to "pruritus" in the "adverse reaction" report.

