Refer to the tables below the questions as you answer the questions.
| Resource | Monographs | Custom Reports | Publisher/Price |
|---|---|---|---|
| DailyMed | *FDA-approved Package Inserts (labels)
*No off-label uses *Manufacturer-specific inactive ingredients are listed |
None | The DailyMed interface is a US government product and is freely available.
The individual package inserts are published by the manufacturers and just made available by DailyMed
|
| Interface/Resource or part of interface | Monographs | Custom Reports | Publisher/Price |
|---|---|---|---|
| Clinical Pharmacology/Tools | ...................... | *Consumer-level interactions report
*Professional-level Adverse reactions report Professional-level interactions report Profesional-level IV compatibility report (based on Trissel's) |
Elsevier/$$ |
| Clinical Pharmacology/Monographs | standard info | ......................... | Elsevier/$$ |
| UpToDate LexiDrug/Tools | .......................... | Professional-level interactions report
Profesional-level IV compatibility report (based on Trissel's) |
Wolters Kluwer/$$ |
| UpToDate LexiDrug/LexiDrugs | *includes drug prices | ............................. | Wolters Kluwer/$$ |
| UpToDate LexiDrug/Martindale: The Complete Drug Reference | *includes English and foreign generic names, scientific names, US and foreign brand/trade names, and CAS registry numbers | ............................... | Wolters Kluwer/$$ |
| UpToDate LexiDrug/AHFS DI | *pearls of information not found elsewhere | ............................... | American Society of Health-System Pharmacists, Inc./$$ |
UpToDate LexiDrug and Clinical Pharmacology contain custom "drug interaction report" tools. However, only Clinical Pharmacology can produce these reports in either consumer-oriented or professional-level language. The UpToDate LexiDrug's "drug interaction report" tool only produces professional-level reports.
To remember that Clinical Pharmacology provides custom consumer-level interaction and adverse reaction reports, think of

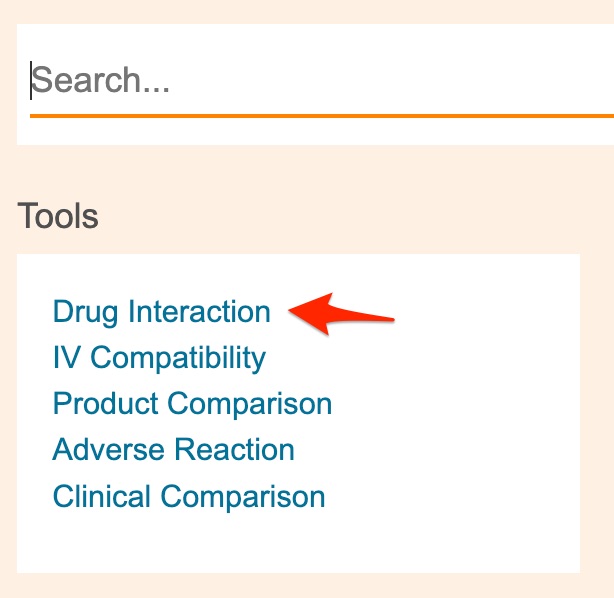
- If you've left Clinical Pharmacology, click here, and pull the new tab/window off of the instruction panel and onto your working window.
- Use Clinical Pharmacology's "Tools" menu to select the "Drug Interaction" option.
If you're on the "Clindamycin" monograph page, the "Tools" menu is available through a link at the top of the page.

If you're on the Clinical Pharmacology homepage, the "Tools" menu is below the search box.
- Enter Mrs. Dilworth's medications (one-by-one) into the "Add drug" box. Mrs. Dilworth's medications include:
aztreonam (solution for injection)
clindamycin phosphate (solution for injection)
risperidone (oral tablet)
ondansetron hydrochoride (solution for injection)
loratadine (oral tablet)
- After entering a drug's name, click the relevant option or hit the keyboard's "enter" key
- When the drug list is complete, select the "consumer" option.
- Hit the "View Results" button.
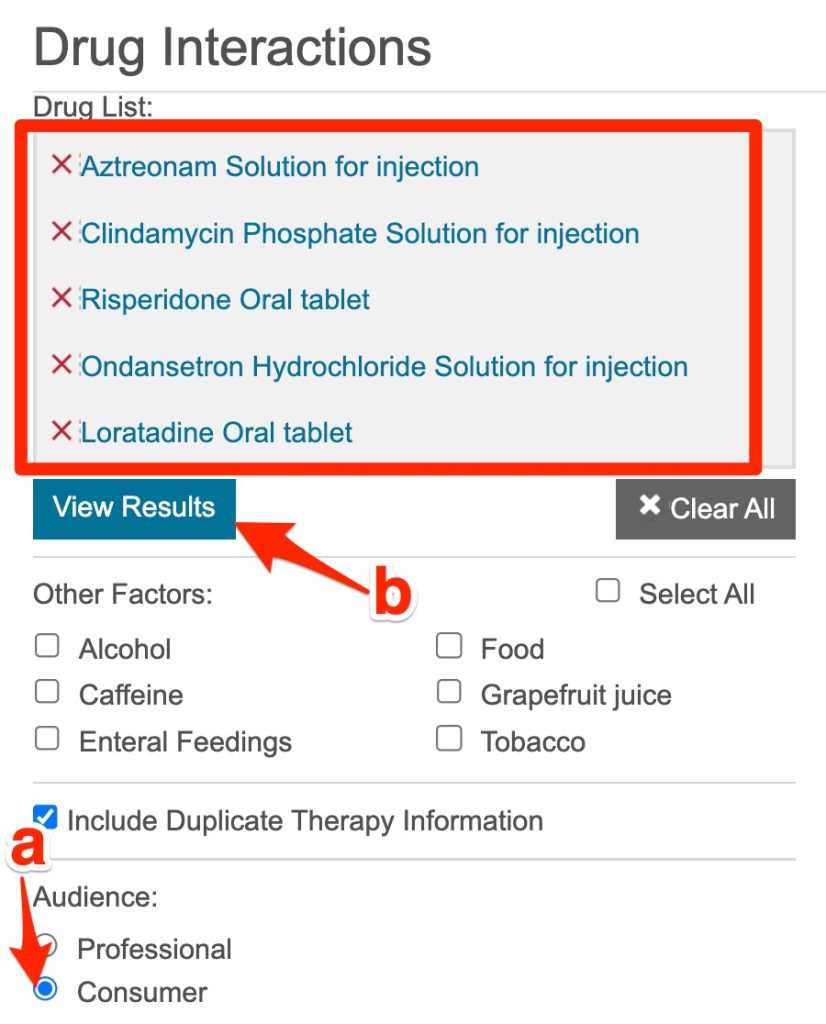
You can change the options (e.g. consumer vs. professional) later by clicking the desired option and hitting the "View Results" button again.

