Two tables summarizing the features of the major, online drug information resources available through the McGoogan Library is shown below.
| Resource | Monographs | Custom Reports | Publisher/Price |
|---|---|---|---|
| DailyMed | *FDA-approved Package Inserts (labels)
*No off-label uses *Manufacturer-specific inactive ingredients are listed |
None | The DailyMed interface is a US government product and is freely available.
The individual package inserts are published by the manufacturers and just made available by DailyMed
|
| Interface/Resource or part of interface | Monographs | Custom Reports | Publisher/Price |
|---|---|---|---|
| Clinical Pharmacology/Tools | ...................... | *Consumer-level interactions report
*Professional-level Adverse reactions report Professional-level interactions report Profesional-level IV compatibility report (based on Trissel's) |
Elsevier/$$ |
| Clinical Pharmacology/Monographs | standard info | ......................... | Elsevier/$$ |
| UpToDate LexiDrug/Tools | .......................... | Professional-level interactions report
Profesional-level IV compatibility report (based on Trissel's) |
Wolters Kluwer/$$ |
| UpToDate LexiDrug/LexiDrugs | *includes drug prices | ............................. | Wolters Kluwer/$$ |
| UpToDate LexiDrug/Martindale: The Complete Drug Reference | *includes English and foreign generic names, scientific names, US and foreign brand/trade names, and CAS registry numbers | ............................... | Wolters Kluwer/$$ |
| UpToDate LexiDrug/AHFS DI | *pearls of information not found elsewhere | ............................... | American Society of Health-System Pharmacists, Inc./$$ |
You should have already opened a working window containing the library's homepage. If not, click here to open McGoogan Library's homepage in a new tab/window. If necessary pull the new tab off of the window containing the instructions and resize the new window so you can see the instructions and new window at the same time.
- Click on the "Resources" menu.
- Click on the "Drug Resources" link.
- Click on the "UpToDate LexiDrug" link.
- You may be asked to login with your UNMC netID (your UNMC e-mail username and password)
The web browser may tell you the website isn't trusted. Go ahead and click buttons/links as necessary to tell the browser that you understand the risks and want to continue or confirm a security exception.
...
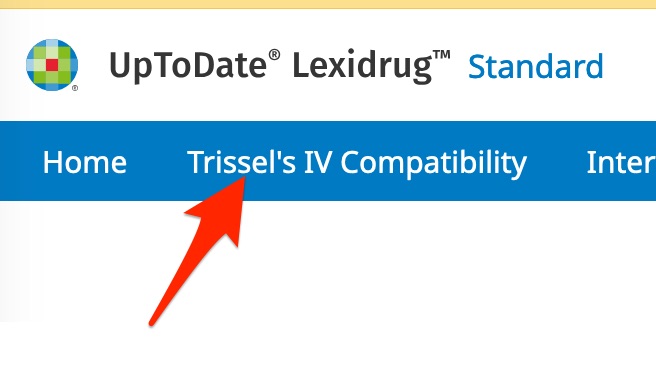
After you reach the "UpToDate LexiDrug" homepage,
- if asked, click "Continue" to allow use of cookies during your "UpToDate LexiDrug" session
- click on the "Trissel's IV Compatibility" button.
You want information on the compatibility of the drugs --
aztreonam
clindamycin phosphate
- Enter the first drug name in the "Drugs" box. One or more forms of the drug should appear below the search box.
- Click on the desired option, or click on the "Add" button.
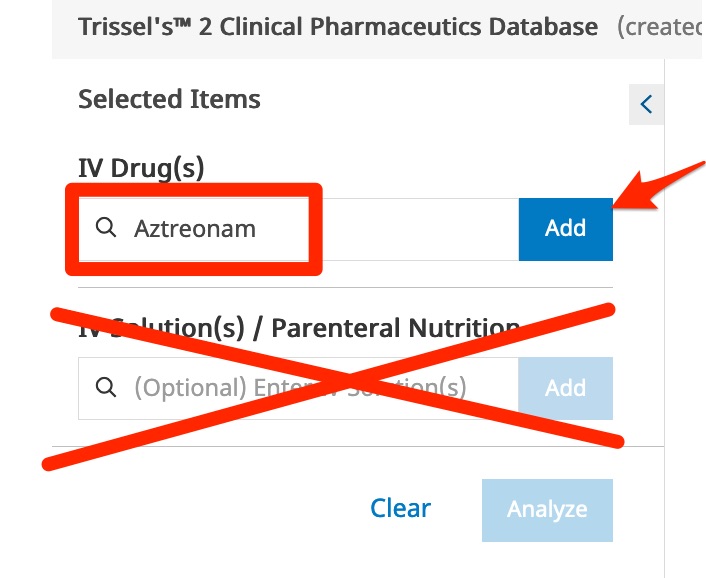
- Enter and add the second drug.
Although you are interested in diluting the drugs in specific solutions, you do not need to enter the solution names here. Entering solutions names here is really only useful if you want information about the solubility of a single drug in a particular solution.
- When both drug names are listed below the "Drugs" box, click the "Analyze" button.
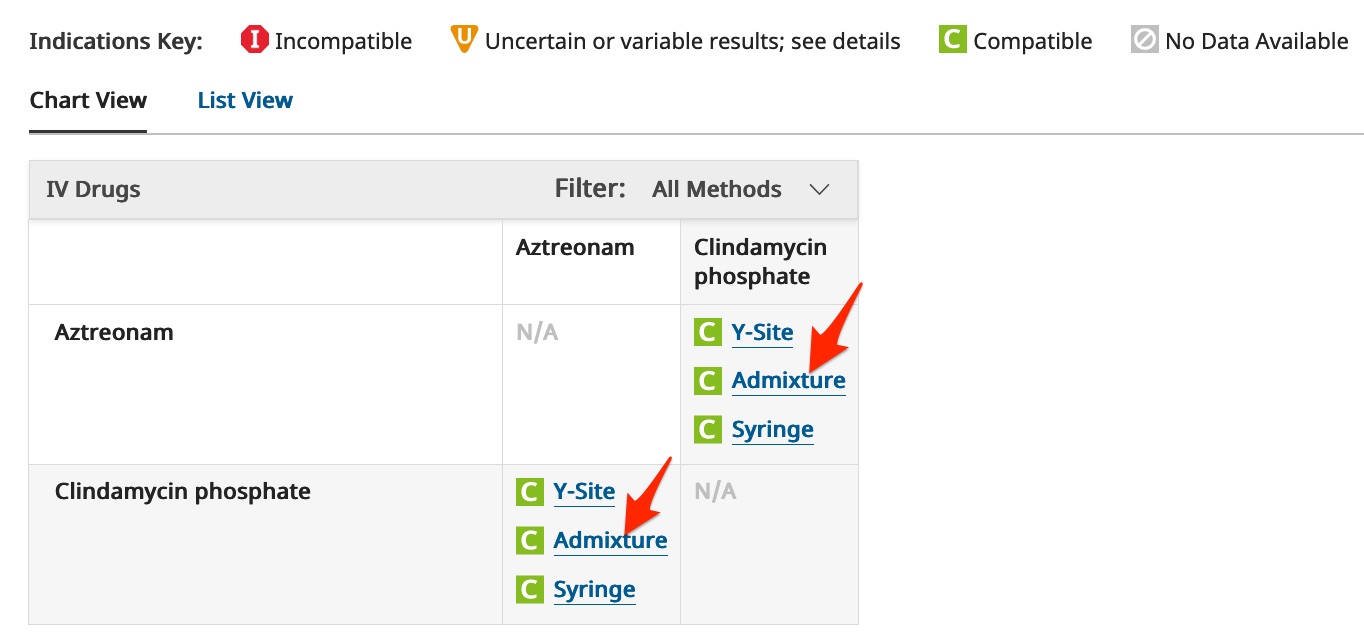
A "Compatibility Chart" will appear. Note the definitions of the icons that appear at the above the chart.
- Click on one of the links to the "Admixture" information to get further details on the different admixture concentrations and diluents that have been tested.
The third-year resident wants to use 10 mg/ml aztreonam and 3 mg/ml Clindamycin Phosphate. Find the entries that include both of these concentrations.
The resident wants to deliver these drug concentrations either in--
Dextran 40 /10% normal saline
(note:dextran and dextrose are not the same thing)
-- or in --
normal saline.
Have the desired drug concentrations been tested for compatibility in either of these solutions?
The resident wants you to show him the journal article that reported the results of the compatibility testing.
When you find the line for 10 mg/ml aztreonam and 3 mg/ml Clindamycin Phosphate in one of the desired solutions, click on either the "Study" link for that combination. You will be taken to more details about the compatibility test.
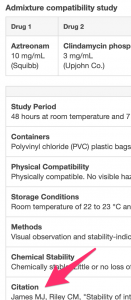
If you're not used to reading journal article citations, the information below will help. Read it and then answer the question.
Here's another citation in the same style as the citation you're viewing.
Marble DA, Bosso JA, Townsend RJ. Stability of clindamycin phosphate with aztreonam, ceftazidime sodium, ceftriaxone sodium, or piperacillin sodium in two intravenous solutions. Am J Hosp Pharm. 1986;43:1732-6.
- The authors names are listed first (Marble DA, Bosso JA, Townsend RJ).
- The article title is listed next (Stability of clindamycin phosphate with aztreonam, ceftazidime sodium, ceftriaxone sodium, or piperacillin sodium in two intravenous solutions").
- The journal title or an abbreviation for the journal title is listed next (Am J Hosp Pharm). Some, but not all, citation styles italicize journal titles.
- The journal title is followed, in this style, by the publication year (1986).
- In this citation style, the next piece of information (43:1732-6) is the volume number (43), followed by a colon, followed by the page number range (1732-6).
It's always best to check at least two sources when answering a clinical question. What if a typing error appears in one of the resources?
Clinical Pharmacology is the other UNMC-licensed resource that provides a custom, IV compatibility report tool.
To access Clinical Pharmacology:
- Click on the "Resources" drop-down menu.

- Click on the "Drug Resources" link.
- Click on the "Clinical Pharmacology" link.
- If asked, select either "University of Nebraska Medical Center, Library" or "University of Nebraska Medical Center, Medical Center" and click the "Continue" button.
To double-check the IV compatibility of aztreonam and clindamycin in admixture:
- Click on the "IV Compatibility" link
- Search for the two antibiotics, aztreonam and clindamycin phosphate, one at a time.
- Click the relevant option after each search.
- When both drugs are present in the "Drug List" on the left, click the "View Results" button.
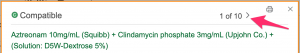
- Since, Mrs. Dilworth's physicians want to infuse the drugs in an admixture, look at the icons in the "Admixture" table
Check the "Legend" at the lower left for icon definitions.
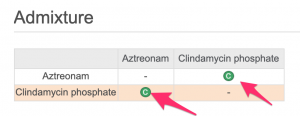
If you want to determine which diluents have been tested :
- Click on a "C" icon in the "Admixture" table.
- A pop-up box will appear.
- Clinical Pharmacology will show one tested dose/diluent combination at a time. If there are 10 tested combinations, you will have to click on the arrow at the upper right of the pop-up to move from "1 of 10" to "2 of 10", etc.