Objectives:
- Understand the concepts:
- (a) adverse effects,
- (b) drug interactions,
- (c) IV compatibility/incompatibility
- (d) major compendium
- Know the unique features of Clinical Pharmacology, UpToDate LexiDrug (formerly known as LexiComp), LexiDrugs, Martindale’s, AHFS DI, and DailyMed
- Be able to access DailyMed, Clinical Pharmacology, UpToDate LexiDrug (formerly known as LexiComp), LexiDrugs, AHFS Drug Information, and Martindale’s
- Be able to find PPIs using Daily Med
- Be able to generate custom Adverse Effects, drug interaction, and compatibility reports using Clinical Pharmacology and/or UpToDate LexiDrug
Pay attention to the stars ⭐
Much of the content presented from this point forward will be represented on your exam and/or on the drug resources quiz. If we were meeting in-person, there are things that I would point out as content that would be sure to be represented on the test. I'm going to use star symbols ⭐ to point out some of this content in the course book. Usually, the test will require that you make a decision based on your knowledge of these pieces of content.
_________________________________________________________________________________
Objective 1 a,b, and c. Understand the concepts "adverse effects," "drug interactions," and "IV compatibility/incompatibility"
You will be using the major drug information resources to look up drugs' adverse effects, interactions, and known IV compatibilities or incompatibilities so it's important that you understand these concepts.
__________________
a. Adverse effects (also called adverse reactions or side effects)
⭐An adverse reaction takes place after the drug's administration to a patient. It is defined as any undesired drug effect. Adverse effects range from mild reactions like
drowsiness

or itching

to more severe and dangerous reactions like suicidal behavior

__________________
b. Drug Interactions
⭐Drug interactions, like adverse effects, take place after a drug is administered to a patient.
Pharmacokinetic interactions
⭐In a pharmacokinetic interaction, a food, beverage, or second drug effects the absorption, metabolism or distribution of the first drug.
Here's an example of a pharmacokinetic interaction:
A patient who takes felopdipine for high blood pressure decides to drink some grapefruit juice. Grapefruit juice inhibits the action of the enzyme (CYP3A4) that degrades felodipine and the patient is exposed to much more of the active drug than he normally would be. This could result in unexpectedly low blood pressure, fainting episodes, and worse.
Take a look at the table below this paragraph. Column 1 lists the anatomical sites at which felodipine concentrations have been measured. Column 2 lists the % of the original dose of active drug still present at these locations if the patient isn't consuming grapefruit juice and column 3 lists the % of the original dose present if the patient has been consuming grapefruit juice. The message is the CYP3A4 activity in the gut and liver will reduce active drug levels much more drastically if not inhibited by the action of grapefruit juice. Thus, the patient who consumes grapefruit juice may be exposed to too much active drug.
| anatomical site | % active drug present when patient is not drinking grapefruit juice | % active drug present when patient has drunk grapefruit juice |
|---|---|---|
| mouth | 100% | 100% |
| after passing through gut lining (which contains CYP3A4) | 30% | 90% |
| blood carries drug to liver | 30% | 90% |
| blood after passing through the liver (which contains CYP3A4) | 15% | 45% |
| blood from the liver returns to the heart and is pumped to the whole body | 15% | 45% |
The information above is shown in pictorial form in figure 1 of https://unmc.primo.exlibrisgroup.com/view/action/uresolver.do?operation=resolveService&package_service_id=1279986780006389&institutionId=6389&customerId=6385&VE=true
Pharmacodynamic Interaction
⭐A pharmacodynamic interaction occurs when two drugs have additive or antagonistic effects.
Here's an example of a pharmacodynamic interaction:
A patient with narcolepsy (a disorder characterized by an extreme tendency to fall asleep) is being treated with a stimulant. The patient has seasonal allergies and is given an over-the-counter antihistamine (with a side effect of drowsiness) by a new co-worker. The antihistamine's side effects diminish the positive effects of the stimulant and the patient falls asleep repeatedly all afternoon.
__________________
c. IV Incompatibility
⭐IV incompatibility, unlike adverse effects or a drug interaction, occurs before drug administration to a patient. It is the result of two drugs reacting with each other or changing the nature of the solution when mixed.
⭐Signs of incompatibility are:
- a change in visible or electronically-determined (even if not visible) particulates, haziness or turbidity, frank precipitation, color, or gas evolution after drug mixture.
- a loss of intact drug >10% within 24 hours when mixture constituents are assayed.
Three types of IV incompatibility
Tests are performed to see if drug mixtures are compatible or incompatible in three different types of mixing situations. The concentrations of the drugs vary in these three situations.
1. admixture
An example of an admixture would be two drugs and a diluent mixed in an IV bag.
In the picture below, preparations are being made to add a drug to the IV bag.

One example of an admixture incompatibility is loss of 50% of one drug's concentration when a mixture of two drugs sits in an IV bag overnight.
2. y-site
The diagram below shows an IV line with a y-site.
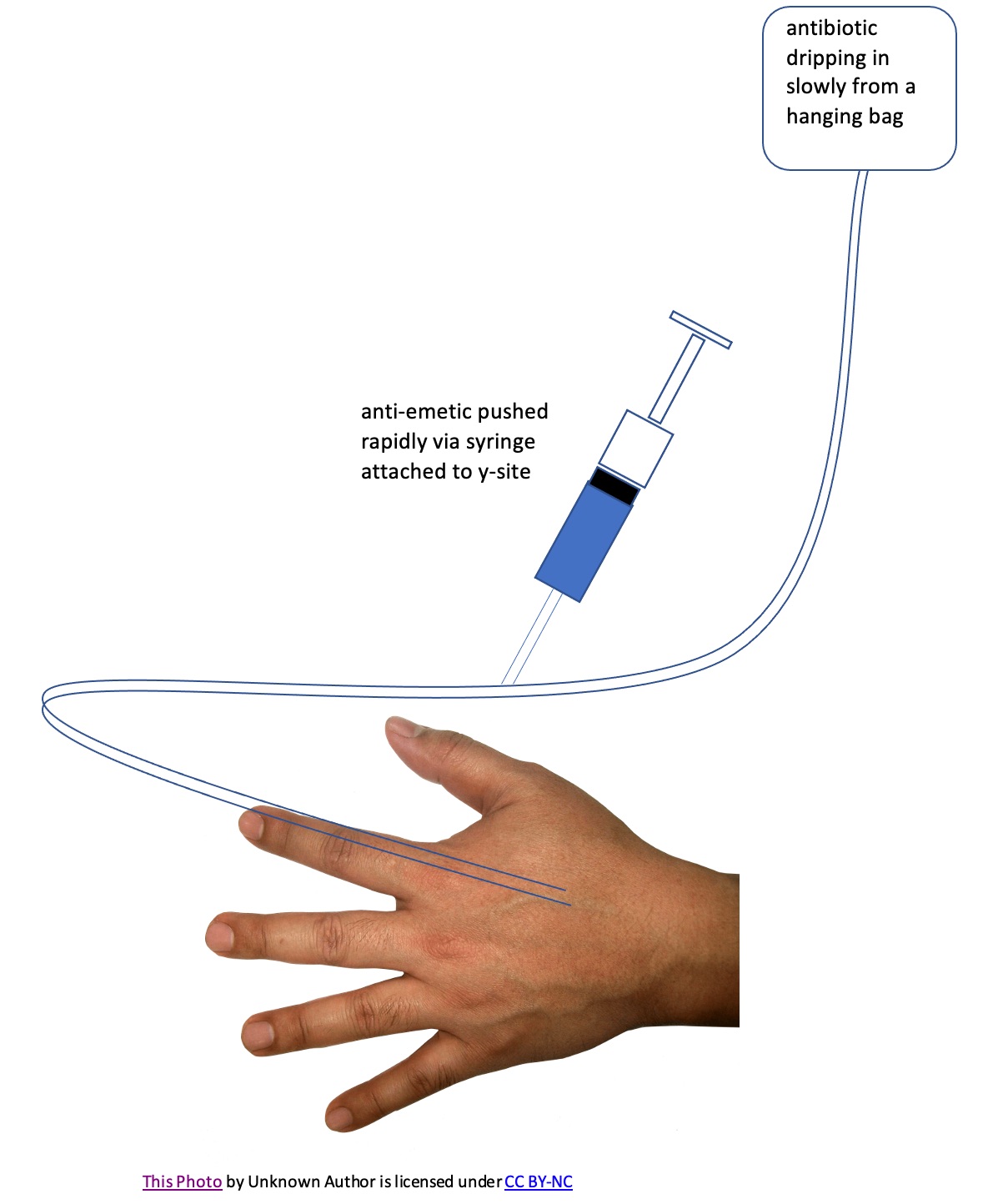
Y-cite mixtures usually involve one drug that is being delivered slowly through IV tubing (often from a hanging IV bag) mixing in the tubing with a second drug when the second drug is administered via a syringe or second IV bag attached to the tubing's y-site.
One example of such an interaction would be formation of a precipitate in the tubing when the two drugs come in contact.
3. Syringe
A syringe mixture involves two drugs that are drawn into a syringe for simultaneous delivery
An example of syringe incompatibility: Two drugs are drawn into a syringe, meet, and the previously clear solutions become milky white.
__________________
Objective 1d. Understand the concept "major compendium"
- Compendium = A collection of concise but detailed information about a particular subject. ·
- A drug monograph is a concise summary of the important information about a drug's properties and therapeutic use
- Therefore, any collection of drug monographs is a drug information compendium
_________________________________________________________________________________
Objective 2. Know the unique features of Clinical Pharmacology, UpToDate LexiDrug (formerly known as LexiComp), LexiDrugs, Martindale’s, AHFS DI, and DailyMed
Two of the drug information compendia available at UNMC, Clinical Pharmacology and DailyMed, are available through their own separate interfaces. UNMC also licenses UpToDate LexiDrug and its native monograph compendium Lexi-Drugs. We pay an extra fee to UpToDate LexiDrug to obtain two additional drug compendia in the UpToDate LexiDrug interface, AHFS Drug Information (AHFS DI) and Martindale's: The Complete Drug Reference.
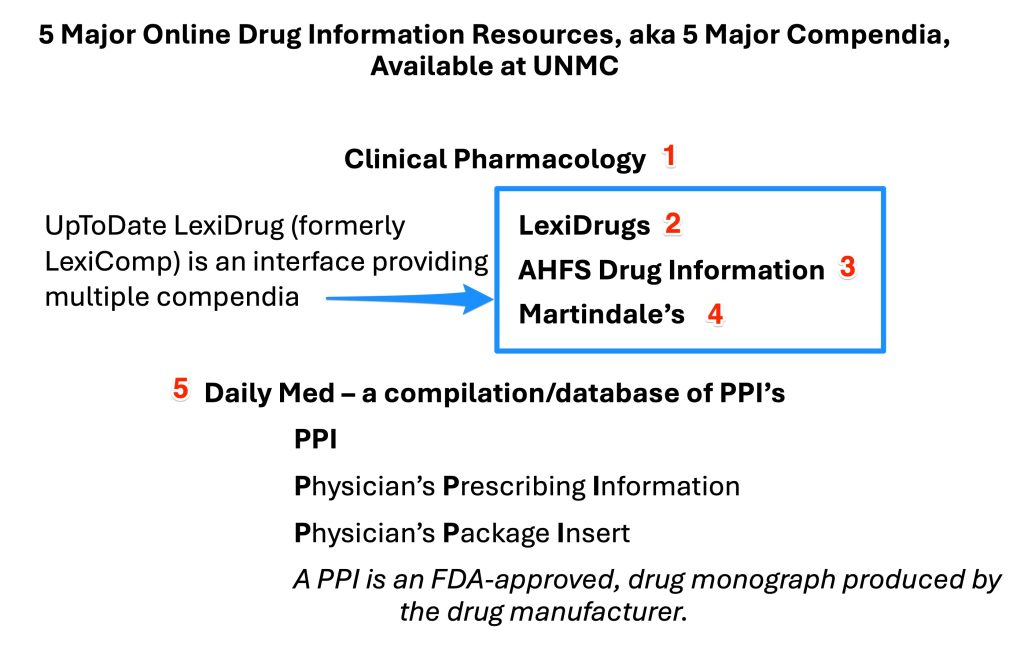
The two tables below provide a summary of the the distinctive characteristics of these major compendia. The distinctive characteristics are shown in italics. Below the table, you will find a more detailed discussion of the distinctive characteristics of these resources and mnemonic devices to help you begin memorizing the distinctives of these compendia.
| Resource | Monographs | Custom Reports | Publisher/Price |
|---|---|---|---|
| DailyMed | *FDA-approved Package Inserts (labels)
*No off-label uses *Manufacturer-specific inactive ingredients are listed |
None | The DailyMed interface is a US government product and is freely available.
The individual package inserts are published by the manufacturers and just made available by DailyMed
|
| Interface/Resource or part of interface | Monographs | Custom Reports | Publisher/Price |
|---|---|---|---|
| Clinical Pharmacology/Tools | ...................... | *Consumer-level interactions report
*Professional-level Adverse reactions report Professional-level interactions report Profesional-level IV compatibility report (based on Trissel's) |
Elsevier/$$ |
| Clinical Pharmacology/Monographs | standard info | ......................... | Elsevier/$$ |
| UpToDate LexiDrug/Tools | .......................... | Professional-level interactions report
Profesional-level IV compatibility report (based on Trissel's) |
Wolters Kluwer/$$ |
| UpToDate LexiDrug/LexiDrugs | *includes drug prices | ............................. | Wolters Kluwer/$$ |
| UpToDate LexiDrug/Martindale: The Complete Drug Reference | *includes English and foreign generic names, scientific names, US and foreign brand/trade names, and CAS registry numbers | ............................... | Wolters Kluwer/$$ |
| UpToDate LexiDrug/AHFS DI | *pearls of information not found elsewhere | ............................... | American Society of Health-System Pharmacists, Inc./$$ |
__________________
A. Resource Published by the Manufacturer under FDA Control: DailyMed
DailyMed is a U.S. government-controlled database. It gives the public an easy way to obtain the FDA-approved package inserts.
- The package inserts are authored and published by the drugs' manufacturers (DailyMed has no responsibility for their content.).
- Package inserts are frequently called physician's or pharmacist's prescribing information, PPIs, FDA-approved monographs, or package labels. ⭐
- Package inserts are approved by the FDA. They can only provide information on FDA-approved drug uses. ⭐
- No uses that have not been approved by the FDA (off-label uses) will be discussed. This does not mean that off-label uses are illegal. Much of the totally legal drug use in the U.S. is off-label.
- The FDA must approve any changes or updates in a package insert. FDA approval of a new label/package insert takes time.
- For the reasons listed above, DailyMed, and package inserts in general, are rarely the best resource for answering drug information questions.
When is DailyMed the best resource?
DailyMed may be the best resource when you need information that is manufacturer-specific.
-
-
- Each manufacturer/repackager must submit its own package label/insert to the FDA for approval.
- Different manufacturers may use different inactive ingredients (excipients) when producing the tablets, capsules, etc. that they distribute.⭐
- Patients are occasionally allergic to ingredients used as excipients, ingredients like lactose or dyes.
- DailyMed can be used to find manufacturers that make a needed drug without ingredients that are a problem for a particular patient. To find the inactive ingredients in a drug preparation:
- Go to DailyMed
- Search DailyMed for the drug's generic or brand name.
- Find one of the results that corresponds to the form of the drug that is of interest (e.g. intravenous, tablet, nasal spray, etc.) and click on the link to the PPI
- "Expand all sections" and use Ctl-f(windows) or Command-f(mac) to open a "find" box. Use the box to search for the word - inactive.
- Keep looking for all instances of the word - inactive. Some package inserts include information about multiple forms of a drug (tablet, capsule, solution for injection, etc.) so be sure you've seen the inactive ingredients for the drug form/preparation of interest to you.
-
DailyMed may also be helpful when you need a chemical structure. Many package inserts include chemical structures.
__________________
B. Resources NOT Published by the Manufacturer and NOT under FDA Control
The resources discussed below can cover off-label (off-PPI, FDA-‐unapproved) therapeutic uses. Authors/Editors can change content quickly, without FDA approval.
If we were meeting in-person, I would ask the class to repeat the mnemonic devices below out-loud. I'd suggest you try saying these out loud and also write them down. It's easier to remember pieces of information if you deal with them in a variety of different ways. The distinctive characteristics of these resources will be covered on the quiz and on the exam. This reflects the importance of these resources in clinical pharmacy practice.
1. Clinical Pharmacology
- Includes drug monographs, a variety of custom report tools and more
- Unique features are:
- Custom, consumer-level interaction (CLI) reports (both LexiComp and Clinical Pharmacology provide professional-level drug interaction reports).⭐
- The word "Clinical" in "Clinical Pharmacology" begins with CLI
- Custom, adverse reaction (AR) reports.⭐
- The word "Pharmacology" in "Clinical Pharmacology" contains AR
- Custom, consumer-level interaction (CLI) reports (both LexiComp and Clinical Pharmacology provide professional-level drug interaction reports).⭐

2. UpToDate LexiDrug (formerly LexiComp)
- Includes a custom drug interaction tool, a custom IV compatability report tool, drug monographs, a variety of custom report tools and more
- Unique feature –unites access to diverse monograph sets in a single interface ⭐
- Think of the "U" and "D" in UpToDate as standing for "unites diverse"

3. LexiDrugs
- LexiDrugs is one of the sets of drug monographs that comes with LexiComp without having to pay an additional fee (on top of the LexiComp license fee).
- Unique features are:
- General LexiDrugs includes a table of drugs that shouldn't be crushed.⭐
- Think of the "X" in LexiDrugs as a symbol for forbidding crushing.
- General LexiDrugs monographs include prices.⭐
- Think of the "S" in LexiDrugs as a dollar sign.
- General LexiDrugs includes a table of drugs that shouldn't be crushed.⭐

(The mnemonic for not crushing drugs "x" "Drugs" is not as obvious as I'd like)
4. Martindale: The Complete Drug Reference via LexiComp
- UNMC pays LexiComp an extra fee to provide Martindale's
- Unique feature: Martindale's provides all the drug names you need to create a very thorough keyword search.⭐
- Think of the word "Complete" in "Martindale: The Complete Drug Reference" as standing for "complete list of names"

5. AHFS DI
- UNMC pays LexiComp an extra fee to provide the AHFS DI.
- Do not confuse the AHFS DI with AHFS essentials.

Advantages of AHFS DI -‐-‐
- Online version is thoroughly referenced.
- Extremely thorough monographs.
- Good source for odd little pieces of info (pearls) like information obtained from conference presentations/abstracts and personal communications with the manufacturer -- Unique feature. ⭐
The mnemonic below is a bit contrived. Think of the letters "A" "H" "F" "S" "D" "I" as the initials on a pearl ID bracelet.

Anticipate Weekly Resource Reviews and Resource Knowledge Assessments
The tutorial and tutorial assignment you will complete today will help you begin to become familiar with these resources.
To help you memorize the resource distinctives, I've added a resource review to the beginning of every week's materials.
You will have a quiz that covers the resource distinctives
The mid-term exam will also cover this material.
_________________________________________________________________________________
Objective 3. Be able to access DailyMed, Clinical Pharmacology, UpToDate LexiDrug, AHFS Drug Information, and Martindale’s
Almost all drug resources available at UNMC can be reached in two ways. Either:
1. You can go to a COP course in Canvas and click the "Library" link in the left-hand navigation column to reach the Library's COP Research Guide with it's list of drug resources on the homepage.
or
2. You can start at the library's homepage
- To reach the library's homepage,
- either google - UNMC library
or
-
- start at any unmc.edu homepage and click on the "Library" link in the list of links at the bottom of the page.
- Click on the "Resources" menu.
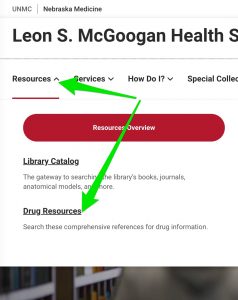
- Click on "Drug Resources"
- Click on the link to the resource of interest.
_________________________________________________________________________________
Tutorial and Assignment
The tutorial and tutorial assignment will give you a chance to practice using the compendia discussed today and will cover the session's last two objectives:
Objective 4. Be able to find PPIs using Daily Med (MA)
Objective 5. Be able to generate custom AE, drug interaction, and compatibility reports using Clinical Pharmacology and UpToDate LexiDrug
Instructions:
- Complete the tutorial at https://pressbooks.nebraska.edu/modi/front-matter/introduction/
- The tutorial contains questions and will provide feedback on your answers.
- After considering the feedback, record your best answer to each of the questions in a document.
- When you've finished the tutorial, return to Canvas and enter the best answers to the questions in the Canvas assignment/quiz entitled: "Major Online Drug Information Resources Tutorial Assignment"
Questions, Problems, Text Errors?
Before you leave, ...
- Do you have any questions or do you feel that clarification of some aspect of the materials would be helpful?
- Have you noticed any errors or problems with course materials that you'd like to report?
- Do you have any other comments?
If so, submit questions, comments, corrections, and concerns to Cindy Schmidt at cmschmidt@unmc.edu.

