An example of a well-written, correctly formatted monograph submitted by a past student provided in this chapter. You may also download the sample monograph if you wish.
_________________________________________________________________________________
Answers to Frequent Student Questions About This Sample Monograph:
- You do NOT need to comment on the color of your drug when it’s in crystalline form or the solubility of the drug in water. It’s fine to include this information, but it is NOT required.
- Students are no longer required to cite a monograph from a printed resource. You do not need a resource similar to #3 in the sample monograph.
- You may cite a source for your structural diagram, but (as shown below) you do not have to do so.
_________________________________________________________________________________
Sample monograph:
Fluoxetine Hydrochloride
Kelsie Post
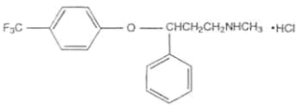
Physicochemical Properties: Fluoxetine hydrochloride is a white to off-white crystalline solid. It has a solubility of 14 mg/mL in water. The molecular formula is C17H18F3NO•HCl, with a molecular weight of 345.79 g/mole. 1
Dosage Forms: Fluoxetine is available in immediate-release oral dosage forms including regular capsules, tablets, or oral solution.1, 2 Fluoxetine is also available as a delayed-release capsule, called Prozac Weekly.2
Administration and Therapeutic Uses: The recommended administration and dosage is dependent on the therapeutic use. Common therapeutic uses of fluoxetine include treatment of major depressive disorder, for which the common adult dose is 20 mg once daily; bulimia nervosa, 60 mg per day in adults; obsessive compulsive disorder, for which the adult dose is 20 mg daily; and panic disorder, where the initial adult dose is 10 mg once daily and increases to 20 mg once daily after one week.1-4
Biopharmaceutical Properties: Fluoxetine is absorbed well within the GI tract, and the presence of food can delay the absorption rate by 1 to 2 hours, but it does not have an effect on Cmax or AUC. There may be some first-pass metabolism that takes place. Fluoxetine is highly protein-bound, 94.5%, to predominately alpha1-acid glycoprotein. Peak plasma concentrations are reached after 6-8 hours with administration of regular capsules and tablets. Fluoxetine has the slowest elimination of the SSRIs, with an elimination half-life of 4-6 days with chronic administration. Primary route of elimination is through metabolism in the liver by demethylation to metabolites which are then excreted by the kidneys. Fluoxetine and its active metabolite, norfluoxetine, inhibit the action of several of the cytochrome P450 isoenzymes.2
Pharmacological Properties: Fluoxetine is a selective serotonin reuptake inhibitor (SSRI). The mechanism of fluoxetine involves binding to the serotonin transporter, blocking the reuptake of serotonin into the presynaptic cell, and therefore increasing levels of extracellular serotonin. When SSRIs are taken for an extended period of time, serotonin autoreceptors are down-regulated, which allows the neuron to increase serotonin release.2,4
Side Effects: The most common side effects experienced with fluoxetine include headache, nausea, insomnia, anxiety, drowsiness, diarrhea, weakness, anorexia, decreased libido, dizziness.1, 2
Drug-Drug Interactions: Concomitant use of fluoxetine and triptans, tramadol, or other serotogenic agents can cause serotonin syndrome. There is an increased risk of bleeding seen in the use of fluoxetine with aspirin and nonsteroidal anti-inflammatory medications. Fluoxetine and its active metabolite, norfluoxetine, inhibit the action of several of the cytochrome P450 isoenzymes, specifically CYP2D6 and CYP384, which therefore limits the metabolism of some other drugs.4 This may lead to an increase in serum concentrations and toxicity of concomitant medications that utilize these pathways. 1, 2
Contraindications: Fluoxetine hydrochloride is contraindicated if the patient is hypersensitive to fluoxetine or any of its formulation components. It is also contraindicated with concomitant use of pimozide, monoamine oxidase inhibitors (MAOI), and thioridazine. Due to the long elimination half-life of fluoxetine, MAOIs and thioridazine should not be started until 5 weeks after fluoxetine has been stopped.1,2
Patient Counseling: Fluoxetine may be taken with or without food. Never stop taking an antidepressant medicine without consulting a healthcare provider. Antidepressant medications may cause worsening of depressive symptoms and increase suicidal thoughts or actions when first started.1 The therapeutic effects of fluoxetine may be delayed for 3 to 4 weeks.4
REFERENCES
- FLUOXETINE- fluoxetine hydrochloride \ capsule. Package Insert. ALPHAPHARM PTY LTD. Updated September 2007. Accessed August 23, 2016. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59de2889-c3d3-4ebf-8826-13f30a3fa439#
- Fluoxetine. In: Clinical Pharmacology. Elsevier/Gold Standard; Updated periodically. Accessed August 23, 2016. https://library1.unmc.edu/login?url=http://www.clinicalpharmacology-ip.com
- Fluoxetine hydrochloride. In: Kastrup EK, Cohn CM, Spenard PL, et al, eds. Drug Facts and Comparisons 2015. Wolters Kluwer; 2015:1634-1637.
- Sawyer EK, Howell LL. Pharmacokinetics of fluoxetine in rhesus macaques following multiple routes of administration. Pharmacology. 2011;88(1-2):44-49. doi:10.1159/000329417

