20 The Road to a Healthy Gut Microbiome
Heather Rasmussen and Sabine Zempleni
Not too long ago, a healthy infant needed to meet two criteria at a checkup: Growing along a percentile anywhere between the 5th and 95th percentile and meeting all developmental milestones.
Breastmilk was promoted as the first choice during the first 4 to 6 months because breastmilk has the optimal nutrient composition for the growing infant. Formula developed at a brisk pace to mimic the nutrient composition of breastmilk and was recommended as an alternative. Introduction of solid foods was recommended between 4 and 6 months.
In reality, breast feeding was not common; it was common to feed solids early and not uncommon that mothers added baby cereal to bottles so the infant slept through the night.
This all changed with the growing understanding of the gut microbiome during the last 10 to 20 years. Today, breast milk is not only seen as the perfect food for healthy infant growth, but the vehicle that enables optimal metabolic and immunological development.
Before you learn how a single food such as breastmilk can accomplish this far reaching physiological impact, you need to review what you already know about the anatomy and physiology of the gastrointestinal tract. And, add a few more details that are important for understanding the development of the gut microbiome.
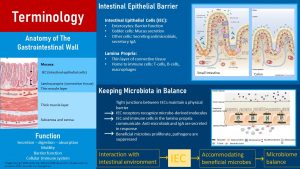
Anatomy: The small and large intestines are in principle a long muscular tube. The wall of this tube is built from several tissue layers. From outside to inside, the first layers are the serosa and sub-serosa, a membrane and a layer of connective tissue (bottom layer in the graphic above, left side). This is followed by several thick muscle layers that allow for the wave-like and constricting contractions, the motility of the GI tract.
The innermost layer is the mucosa which is built by three sub-layers: A thin muscle layer, a layer of connective tissue (lamina propria), and the intestinal epithelial cells (IEC). The IEC and lamina propria form the functional barrier between body and the lumen of the intestine.
The main cell type of the IEC are the epithelial cells. Inserted between the epithelial cells are Goblet cells. Goblet cells secrete protective mucus and are involved in repair and maintenance of the IEC. a good layer of mucus ensures that the ut epithelium is not digested. Several other cell types are also embedded. Their function is to secrete antimicrobials, hormones, and IgA.
The connective tissue (lamina propria) below the IEC contains immune cells. These include macrophages, part of the innate immune system, and cells of the adaptive immune system (dendritic cells, B- and T-cells). The IEC and the immune cells communicate with each other about signals from the gut microbiome but also signals sent from the brain, metabolism, and immune system.
Barrier Function: The IEC is formed by a single layer of cells. These cells are not just lined up closely next to each other, but two cells are connected by the so called tight junctions (image bottom right of the infographic above). Tight junctions are built by several proteins lined up between cells so the IEC forms a physical barrier between body and GI tract lumen. Microbes and larger nutrients are kept in the gut lumen.
Keeping the gut microbiome in balance: The trillions of commensal bacteria are essential for the ongoing education of the immune system in the infant, but they also pose a risk for inflammation.
The barrier function of the mucosa is one way to keep microbes out of the body, but the interaction between the gut mucosa, the gut microbes, and the immune system goes much further.
Receptors on the IEC cells detect microbe-derived molecules and signal this information to the immune cells in the lamina propria and the secretory cells of the IEC. IgA is secreted in response and transported through the epithelial cells into the gastrointestinal tract lumen. At the same time IEC cells secrete anti-microbials.
This chemical communication allows the beneficial commensal microbes to thrive while suppressing pathogens and unwanted microbes. This complex mechanism keeps the gut microbiome healthy and in balance.
You might have noticed that I keep referring to beneficial or wanted microbes. What are beneficial bacteria? How does the ICE and immune system know which microbes are beneficial and which microbes need to be suppressed? What gut microbiome composition is connected to a healthy immune system and metabolism? Why do some people have a healthy and gut microbiome while others have an unhealthy microbiome?
You will learn:
The development of the gut microbiome takes place throughout the first three years of life, the so called window of opportunity. After birth the gut microbiome increases gradually in amount and diversity. Goal is a diverse and resilient gut microbiome. The ideal circumstances are:
- Delivery: Vaginal
- First six months: Breast milk
- Introduction of solids: After 4-6 months
- Weaning: After 12 months
- Solids, family meals: Plant heavy, rich in indigestible carbohydrates
The immune system develops gradually along with the gut microbiome. The goal is a homeostatic, resilient and vigilant immune system. The steps of the gradual development are:
- Stimulation of the innate immune system at birth while suppressing the adaptive immune system
- Phase of immune tolerance until 3 month to allow for the development of the innate immune system and barrier function of the gut mucosa
- Stimulation of the adaptive immune system with introduction of solids and reduction of breast milk
If the difference between the innate and adaptive immune system has become foggy, go back to the first chapter Obesity Alters the Metabolism and review the immune system text box.
The Gut Microbiome Is Connected to Health and Wellbeing
If you would have taken this course twenty years ago, you would have learned that the gut microbiome in the colon is just an afterthought of human digestion. The main functions were thought to be digesting leftover nutrients and some of the indigestible fiber, keeping us regular, and producing some vitamin K, biotin, and a small amount of short chain fatty acids.
Those three functions still apply, but functions of the gut microbiome expanded enormously. Additional functions are the maintenance of the gut barrier, development and regulation of the immune system, and regulation of the metabolism.
During this last decade, scientists also realized that people suffering from several chronic diseases or mental health issues are marked by an out-of-balance gut microbiome called dysbiosis. Many studies demonstrated that obese individuals have a different gut microbiome composition than lean individuals.
Gut microbiome research, a very popular field of research, is just beginning. At the moment, there are more questions than answers. Here are a few broad hypotheses that are discussed regarding the interaction of gut microbiome and health.

Immune System: The gut microbiome is closely connected to the immune system. The immune cells in the lamina propria are responding to composition and fluctuations of the gut microbiome. This local immune system is then connected to the overall immune system in the body. Through this, an unhealthy gut microbiome composition is connected to an increased risk for immune-related conditions such as asthma, allergies, and auto-immune conditions.
Chronic systemic Inflammation: An unhealthy microbe composition increases chronic, systemic inflammation, which is one of the contributing factors to chronic diseases such as T2D and CVD.
Gut-Brain Axis: Think back to the HPG axis you learned about during the pre-conception and pregnancy chapter. This metabolic concept centered around the brain as the regulatory place for metabolism and physiologic functioning. The gut-brain axis connects the microbiome to those regulatory centers in the brain using neurological and endocrine signals. Together, microbiome and brain collaborate on regulating many body functions.
The mediators of the gut-brain axis respond, on one side to environmental cues, including nutrient composition of food and metabolites produced by the intestinal microbiome. The dietary pattern consumed can modulate the gut microbiome, which in turn can affect the metabolism, but also mood, even depression and anxiety. However, this regulation is a two-way street. Stress, a shift in hormonal regulation, or neurological changes can lead to diarrhea, IBS (irritable bowel syndrome) and a general change in the gut microbiome composition.
Obesity: Based on the current scientific evidence we understand that obese individuals have a different gut microbiota composition than lean individuals. This altered microbiota composition increases systemic inflammation and the risk for chronic diseases. At this time, it is not clear what comes first though. Does obesity change the gut microbiome or does an altered gut microbiome enable obesity. Or, are both interacting and aggravating the risk.
The question is now why some people have a healthy, stable, and resilient gut microbiome while others don’t.
Does every healthy person have the same gut microbiome?
In adults, 90 % of the microbiome is made up by the two phyla, Firmicutes and Bacteroitedes. Each phyla includes hundreds of different bacteria strains. The quantitative relationship of those two phyla—the F/B ratio—and the diversity within each phylum is established during the first 3 years of life. After that window, the composition is fairly constant, but can be impacted by lifestyle, moving to a different geographic location, age and antibiotic use. As a consequence, each individual has a unique microbiome profile.
A main factor determining gut microbiome difference between individuals is the living environment including the diet. For example, people living in a non-Western environment and eating a diet rich in complex carbohydrates and fiber tend to have a gut microbiome that is more diverse and marked by prevotella (part of the bacteroitedes phyla). In contrast, people living a Western lifestyle tend to have little prevotella and more bacteroides plus less diversity. Even within a region there will be differences between ethnic groups.
What is a healthy microbiome?
Once a child is 3 years old the microbiome is established and relatively stable. At this time, the IEC and the immune cells have establishe a memory for which microbes to promote and which microbes to suppress. While the research is still very new and incomplete, and the overall field is incredible complex, scientist have identified general microbiome patterns that are connected with better weight maintenance and a lower risk for chronic diseases. Scientists also think that some microbiome patterns are more resilient when exposed to antibiotics, diseases, or aging. These desired patterns tend to have a greater diversity of bacteria as well as less firmicutes and more bacteroitedes.
What is dysbiosis?
Dysbiosis is an unhealthy gut microbiome composition and is linked to increased inflammation, a weak mucosal barrier—the often mentioned leaky gut—and a misregulation of the gut-brain axis. High amounts of firmicutes, less bacterial diversity, and more instability are thought to be hallmarks of dysbiosis. However, it should be noted that, while these attributes are often associated with dysbiosis, the exact composition of the microbiota in the state of dysbiosis has not been delineated. Dysbiosis is thought to be connected with an increased energy production by the microbiome from fiber, more chronic inflammation, weakened tight junctions between cells, colon cancer, auto-immune diseases, T2D, Alzheimer’s, depression, stress and many more.
The Adult Gut Microbiome Is Set Up During The First Three Years of Live
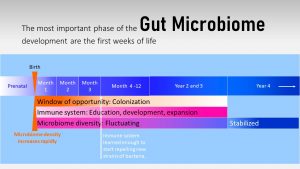
Before birth, the fetus lives in an almost sterile environment protected by the placenta and uterus. However, there is some research pointing to the fact that the fetus might be exposed to small amounts of the mother’s gut microbiome or the genetic material and microbial stimuli produced by the maternal microbiome. This is a topic of discussion between rsearchers, but even if the fetus is exposed to small amounts of microbes, it is unclear if this has an impact on the microbiome development.
During and right after birth, the fetus becomes fully exposed to environmental microbes. Keep in mind, that a hospital will have different bacteria present than a home, a rural location will be different than an urban area, and different geographical locations will have vastly different microbes in the environment. Depending on the delivery mode and the environmental bacteria, the infant develops a starter microbiome. This starter microbiome will undergo gradual compositional changes during the first 2 to 3 years.
Along with the developing gut microbiome, the immune system starts learning, developing and expanding. So far we thought that the immune system is immature when the infant is born. Today, the evidence points more and more to the fact that the immune system of a newborn is designed in a specific way to allow environmental microbes to culture the gastrointestinal system and all other body surfaces in contact with the environment.
This time between birth and an age of 2 to 3 years when the gut microbiome is established and the immune system is educated is called the window of opportunity.
After birth, the immune system is permissive to new bacteria strains for about three months. During this time, the immune system learns to identify what types of bacteria are beneficial and which ones needs to be repelled. As you will see later, the gut microbiome and immune system will get lots of help from human milk during this learning process.
During those three initial months, the infant is very vulnerable to ingested pathogens. The immune system, permissive to new microbes, cannot identify pathogens properly. This is a risky time for gastrointestinal infections especially when feeding infant formula with a bottle. Proper sanitation of equipment is essential.
After three months the window of opportunity starts to close slowly. It becomes more difficult to establish new bacteria strains in the gut microbiome. Changes of microbiome composition—which strains proliferate better than others—is still seen until the end of the second to third year. These changes are now predominantly fluctuations in the composition of established microbes.
If a healthy, diverse and resilient microbiome is established between 2 to 3 years of age, it will remain mostly stable through adulthood. This also means that it will become very difficult to establish new strains of bacteria to improve gut microbiome composition.
An unhealthy, unstable gut microbiome in adults is vulnerable to gastrointestinal infections and dysbiosis after antibiotic use. The effect seems to be more pronounced the less diverse and less stable the microbiome is at the closing of the window of opportunity.
The Microbiome Develops In Phases
At this point you know the big picture. During the next section you will understand the phases of microbiome development better. Keep in mind that the research is evolving. While we understand the big concepts, research to understand the details and intricacies is ongoing.
The first three years of life are called the window of opportunity which lasts until age three give and take. During this time the infant’s gut microbiome is highly sensitive to the environment. Environmental changes can easily alter the gut microbiome composition.
The window of opportunity closes around age 3 when the toddler has developed a highly stable adult gut microbiome. The adult microbiome has only minor fluctuatios and can only be altered by medical conditions, antibiotic use or major, persistent diet changes.
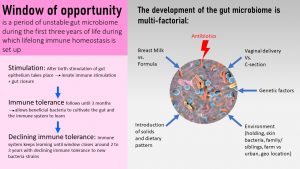
At Birth, Infant And Microbes Meet
During vaginal birth, the newborn experiences the transfer of an immense quantity and diversity of microbes from the maternal microbiome of the vagina and partially the gut microbiome. Handling the newborn will add skin and environmental bacteria to the mix.
If an infant is born via c-section, the transfer will come predominantly from skin contact and will resemble more environmental bacteria. The result is a completely different starter.
This first colonizing by microbes during and right after birth initiates the innate immune system in the gut. The activation of the innate immune system is accompanied by an immune tolerance by the adaptive immune system. The immune tolerance of the adaptive immune system allows for the colonization of the intestines.
The young infant is protected though. At the end of the pregnancy, immune globulins to common environmental pathogens are transferred from the mother to the fetus. Additionally, high amounts of secretory IgG and IgA are supplied by colostrum. Mature breastmilk will keep providing IgA and antimicrobials to suppress pathogens in the gastrointestinal tract of the infant. Breastmilk-fed infants are therefore exposed to more protective immune compounds than formula-fed infants.
During the First Months Bacteria Strains Compete For a Spot in the Gut Microbiome
After birth, the amount of bacteria in the infant’s colon is still relatively low compared to that of an established microbiome. The initial lack of gut microbiota in the newborn allows bacteria to compete for space and nutrients. While genetic factors shape about 9% of the microbiome, the majority is shaped by the infant’s environment.
Type of milk: The nutrient composition of the milk determines what bacteria strains will thrive. Breast milk is lactose rich and contains specific nutrients—oligosaccharides, lipids, peptides—to promote a very distinct bacterial composition and rapid colonization.
Formula fed infants lack this “educational support” and have a different, potentially less favorable gut microbiome composition.
Environment: Another factor influencing the microbiome composition is the transfer of skin bacteria when the infant is held or nursed. Environmental bacteria are also transferred from toys, clothes, bed, and changing table; in short, everything the infant comes into contact with. The bacteria strains vary by geographic location or if the infant is living in a rural or urban area.
Since the adaptive immune system is permissive during the first 3 months, pathogens can also establish themselves easily. This makes it important that feeding equipment is sterilized and the infant is kept from exposure to individuals experiencing infectious diseases.
When rapid spread of infectious diseases is likely, for example in a daycare setting, regular sanitizing of toys will prevent the rapid spread of infectious diseases between children. However, this does not mean that every item the infant comes into contact with at home needs to be sanitized. Transfer of some micro-organisms should be allowed.
Researchers have found that infants growing up in an environment with rich environmental bacteria, such as infants in rural settings or in households with animals, children have a lower risk for allergies than children growing up in highly sanitized urban settings.
Today, it is thought that the approach of allowing rich bacteria transfer will promote development of robust immunity, one that is reliably protecting the infant but not over-stimulating the immune system. Failure to properly prime the immune system may lead to a misdirected response and may result in allergies and autoimmune diseases.
Antibiotics: The younger the infant, the easier the gut microbiome can be disrupted by antibiotics. As antibiotics reduce beneficial microbes and pathogens, antibiotic use might allow less desirable bacterial strains to establish themselves and persist.
During the First Four to Six Months Breastfed Infants Have a Once-In-a-Lifetime Gut Microbiome
Human milk has clearly a pivotal role in establishing a healthy gut microbiome. Think back—or review—the chapter about the nutrient composition of breast milk. Human milk has an array of nutrients that will help establish a very unique gut microbiome.
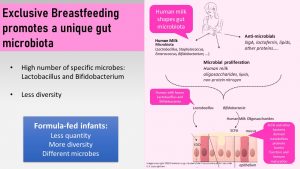
What makes the gut microbiome during breastfeeding so unique?
- High number of microbes and low diversity
- The dominant strains are lactobacilli and bifidobacteria.
The human microbiome will only have this very unique composition during this short time of life when the infant is exclusively breast fed and no solids are fed. It is hypothesized that this composition educates the immune system optimally and guides into the next phase when solid foods are added.
Human milk has three ways to ensure that this specific microbiome establishes during the time of immune tolerance:
- Human milk microbiota: The human milk contains an array of bacterial species such as bifidobacteria, lactobacilli and many more. Sources of these milk bacteria are the infant’s and mother’s mouth, skin, breast, and bacteria from the maternal digestive tract.
- Antimicrobials: Secretory IgA (immune globulins), lipids, lactoferrin and peptides work as antimicrobials and protect from pathogens while promoting the specific strains of bacteria.
- Nutrients for bifidobacteria and lactobacilli: The oligosaccharides in human milk (HMO) are indigestible to the infant’s digestive system but serve as food for the gut microbiome. Every oligosaccharide requires a specialized enzyme or set of enzymes to be digested. HMOs match up with the digestive enzymes of bifidobacteria and lactobacilli. This means that bifidobacteria and lactobacilli will find ample food in the gut of a breastfed infant which allows them to proliferate while other bacteria strains that are not equipped with these enzymes will not be able to grow. The suppressed bacteria are still present in the breastfed infant’s gut—keep this in mind for later in the chapter—just in smaller amounts. Other nutrients that promote these two strains of bacteria are certain lipids and non-protein nitrogen.
Promoting this very specific gut microbiome during the time of immune tolerance seems to mature the barrier function of the gut epithelium and develop a healthy, resilient immune system.
The gut microbiome of infants fed formula looks very different. Formula-fed infants have a lower quantity of microbes, lack the unique bifidobacterium and lactobacillus composition, and have a lower diversity of microbes. Limited evidence indicates that breastfed infants have less allergies and are at a lower risk for chronic diseases later in life compared to formula-fed infants.
While we start to understand how breastmilk feeding is modifying the gut microbiome and educating the immune system, more research is needed to say with certainty how much type of feeding impacts health later in life.
The Gut Microbiome Needs to Be Developed Gradually: Next, Introduction Of Solids And Family Foods
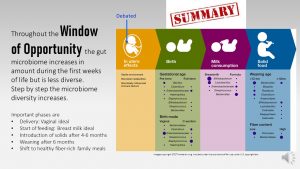
The third phase on the road to a healthy gut microbiome is the introduction of solids. Even if mothers transition to solids after 6 months, the complete weaning from human milk is not recommended until 12 months.
Feeding solids—this should include cereals, vegetables and fruits—introduces fermentable fiber to the microbe community. These indigestible carbohydrates provide food to microbes that were so far present but suppressed due to their inability to digest HMOs. Finally having ample food these microbiota now thrive and enhance the microbiome established by human milk consumption.
The graphic above demonstrated how different feeding decisions can profoundly change the gut microbiome composition. The ideal composition is reached when parents feed a pattern rich in plant foods and keep feeding breastmilk until the end of the first year.
Once the child joins the family meals after the first year (the window of opportunity is closing but still open), many toddlers raised in Westernized countries are subject to diets high in fat, animal protein, sugar, and starch. Researchers are concerned that the Western dietary pattern lays the last stone for a less diverse and resilient microbiome.
Overall, we largely understand the important factors for gut microbiome development, but science is still far away from predicting exactly how deviating from this ideal influences health status.
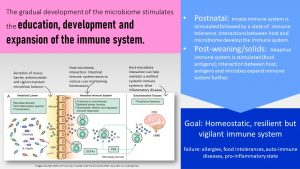
The Gradual Development of the Microbiome Is in Sync With the Development of the Immune System
The next question we have to ask ourself is why the infant gut microbiome is so volatile while the adult gut microbiome is highly stable. The key to this is that along with the gut microbiome the immune system develops in phases as well. In the adult gut microbiome the immune system has a very rigid idea what bacteria are supposed to be there and which ones are not. Healthy composition or not the immune system makes sure the composition stays stable.
Let’s think this succession of microbiome development through with the focus on immune system development.
The innate immune system is triggered during the delivery of the infant. At this point, the adaptive immune system is suppressed and protection to common diseases comes from the secretory immune globulins transferred from the mother to the fetus shortly before birth.
During the first days of life, colostrum provides secretory IgG which can pass through the gut epithelium and protect the infant, for example, against respiratory illnesses. After the first few days, gut closure takes place and secretory IgG can no longer pass through the gut epithelium.
The main protection will now come from secretory IgA in mature human milk that protects from gastrointestinal infections.
The unique immune system of the infant and the protection provided by the mother allow for a time of immune tolerance during the first three months. The immune tolerance is essential to allow for the establishment of the barrier function of the gut mucosa and the development of the immune system. The immune system is introduced to common commensal bacteria strains and learns to identify them as beneficial.
Feeding breastmilk exclusively keeps the exposure to antigens low. Once solid feeding starts, the variety of foods increases exposure to food antigens. The antigens stimulate the adaptive immune system, which starts learning about friends and foes. If this stimulation happens too early, before the infant is four months old, the adaptive immune system might become overactive. The risk to develop allergies and food intolerances increases.
Lately, the recommendation for infants with a allergy predisposition changed. If the infant has not yet developed a food allergy common allergenic foods should be fed along with other solids. In the past the recommendation was to wait introducing foods with a high potential for allergies. Recent research showed though that waiting increases the risk to develop an allergy.
This sequence of events—four to six months of exclusive breastfeeding, then a plant-heavy combination of solids plus human milk, to family meals with a composition of the MyPlate recommendations (Dietary Guidelines for Americans)—seems ideal. This way a homeostatic, resilient, and vigilant but not too overactive immune system is developed.
What Happens If the Microbiome Is Not Diverse and Resilient?

Depending on the combination of ethnicity, geographic location, delivery mode, milk feeding, environmental bacteria, timing of solids, timing of weaning, antibiotic use, and eating pattern, every person develops a very individual gut microbiome during those first three years. It is not clear what the perfect gut microbiome would look like or if it even exists.
What is clear is that some people have a gut microbiome that is less diverse, less resilient, and overall imbalanced. These less resilient microbiomes are less likely to fully bounce back after gastrointestinal infections or antibiotic use. The failure to bounce back can make space for the proliferation of pathogens or lead to an imbalance called dysbiosis.
As described previously, dysbiosis indicates an altered microbe composition that does not function properly in maintaining immune system balance and the mucosal barrier function. Dysbiosis is linked to increased inflammation and a weak mucosal barrier—sometimes called leaky gut—due to impaired tight junctions. The changed chemical and neural signaling modify the metabolism, increase the risk for chronic diseases, or impact even mental health via a disturbed gut-brain axis.
Can you fix dysbiosis?
You probably have read about prebiotics and probiotics that are marketed to fix a leaky gut or dysbiosis. Does it work? First things first, what is the difference between prebiotics and probiotics?
Prebiotics are the nutrients bacteria strains need to thrive. Prebiotics are found in plant-based foods and include fermentable fibers, including oligosaccharides, as well as other plant-derived compounds. A diet high in animal based protein, fat, refined flour products and sugar, aka the Western diet, are often low in prebiotics. Increasing prebiotics in the diet by eating lots of whole grains, legumes, fruits and vegetables will shift the gut microbiome to a more beneficial composition.
Probiotics are live bacteria. They can either come from fermented foods in the diet or from a supplement. Familiar fermented foods include yogurt, fermented vegetables such as saurkraut and kimchi, kombucha, miso, temphe, fish sauce, and salami. Every culture tends to have their own set of fermented foods. The farther North you go, the more fermented foods you will find in the food culture. This can range from fermented fish and meats, cheeses and a variety of fermented cold climate vegetables. If you visit a grocery store specializing in foods from Asia, you will find entire fridges filled with fermented vegetables, fruits, cereals, legumes and soy products. Fermentation of food is used to preserve foods. The bacteria strains in fermented foods tend to produce lactic acid, preserving the food and giving the food its tangy flavor. Lactobacillus, Streptococcus and Bifidobacterium strains thrive in the acidic environment and suppress pathogens that do not.
Does consumption of fermented foods change the microbiome composition? The problem is that the bacteria in fermented foods are well adapted to the acid environment in the food, but not to the GI tract. In food they have ample amounts of custom nutrients and little competition. In the GI system preferred nutrients are not available and competition is large. If they survive transit, they do not become permanent, stable members of the gut microbiome after just one meal. To have a health benefit, those fermented foods need to be part of the regular diet. Researchers hypothesize that probiotics increase gut microbiome health by:
- Producing anti-microbial substances
- Competing with pathogens for nutrients and adhesion to the epithelial cells
- Stimulating positive modulations of the immune system
- Inhibiting toxin production
Studies showing health benefits of fermented foods are mostly observational and conducted in populations who traditionally eat those fermented foods. At the moment there is little evidence available if a new habit of eating fermented foods can reduce chronic diseases. The health benefits of fermented food might come from the microbes or the nutrient density of the foods.
What about probiotic supplements?
Per the FAO (Food and Agriculture Organization), probiotics are defined as Live strains of strictly selected microorganisms which, when administered in adequate amounts, confer a health benefit on the host. Studies are currently underway evaluating the use of probiotic supplements, sometimes in combination with prebiotics (synbiotics), as an treatment option for IBD, Crohn's disease, allergies, dysbiosis after use of antibiotics, and antibiotic induced diarrhea. Lately this line of research was expanded to obesity, insulin resistance, depression and anxiety, but human studies are at the moment lacking. Probiotic supplements contain one or more selected microbial strains similar to the strains in fermented food. Examples are bifidobacterium and lactobacillus, as well as other lactic acid producing strains.
The research results are promising but there is still a long way to go. We do not know what the ideal gut microbiome composition is. Results for one specific strain or combination of strains cannot be transferred to other strains or combinations. Most studies researching and developing probiotic supplements are conducted in Western countries, often in Caucasian populations. Some scientists point out that the probiotic supplement would need to be adjusted to different ethnic groups and not be one-size-fits-all. Probiotic supplements are able to establish a temporary microbiome change, but once the person stops taking the supplement, the immune system remembers which bacteria are allowed and not allowed, and the gut microbiome reverts back.
Even if you hear that a probiotic is scientifically developed and that physician-approved probiotic supplements might reduce symptoms of gut inflammation, bloating, and diarrhea, they are not a permanent fix. Until science makes more progress, the best option is a pre- and probiotic rich diet.
All this makes it very clear that the first three years of life are crucial for the development of a balanced and resilient gut microbiome and immune system. Even if not everything goes as planned, parents have it in their hands to set up a healthy metabolism for their child by getting as many feeding decisions as possible right during these important early years of life.
Interested? Want to Know More?
Probiotics and Prebiotics: https://www.worldgastroenterology.org/guidelines/probiotics-and-prebiotics/probiotics-and-prebiotics-english
Overview Dysbiosis: Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219-232. doi:10.1038/nri.2017.7
IgA or immunoglobulin A is an antibody of the adaptive immune system that plays a crucial role in the immune function of mucous membranes.
Part of the adaptive immune system: Present antigens to B- and T-cells
T and B lymphocytes (T and B Cells) are involved in the acquired or antigen-specific immune response. They memorize, recognize and respond to specific antigenes.
Intestinal epithelial cells
The hypothalamic–pituitary–gonadal (HPG) axis is responsible for regulating reproductive activity in men and women.
In biology, a phylum is a level of classification or taxonomic rank below kingdom and above class.
The phylum Firmicutes includes gram-positive bacteria that are predominantly from the genera Bacillus, Clostridium, Enterococcus, Lactobacillus, and Ruminicoccus
The phylum Bacteroidetes includes species of Gram-negative bacteria that are predominantly from the genera Bacteroides, Provotella, Alistipes, and Parabacteroide.
What is it? See chapter 1 "Quick Biology Review"
Immunoglobulin G is the main antibody found in blood and extracellular fluid. The function is to control infection of body tissues.
Human milk oligosaccharides
Components in food, mostly proteins, that can be recognized by the adaptive immune system and have the potential to trigger allergies
Irritable bowel syndrome (IBS) is a gastrointestinal disorder that affects the large intestine. Symptoms include cramping, abdominal pain, bloating, gas, diarrhea or constipation, or both.
Crohn's disease is a severe type of inflammatory bowel disease (IBD). It is marked by inflammation of the digestive tract, which leads to waves of abdominal pain, severe diarrhea, fatigue, weight loss and malnutrition.


Feedback/Errata