8 Weight Is The Main Nutritional Factor Influencing Fertility
Sabine Zempleni and Sydney Christensen
During the last few decades, concerns have grown about the increasing male infertility. Worldwide studies show that in most countries, sperm count has been declining by 57% over a span of 35 years. Only a couple of countries, such as Australia, do not show this decline.
It is not entirely clear what lifestyle and environmental factors trigger this decline. Of course, scientists are busy at work looking into the reasons. One factor may be the Western lifestyle, which involves consumption of processed foods, sedentary behavior, and chronic stress. Other factors such as obesity and endocrine disruptors may play a role. Endocrine disruptors are environmental chemicals—some insecticides, fungicides, surfactants, plastics, dioxin, and DDT—that are thought to modify hormonal secretion.
Female fertility is being affected as well. We are seeing an increasing prevalence of PCOS (polycystic ovary syndrome) the leading cause of infertility in women. PCOS causes hormonal imbalances which prevent ovulation, and thus prevent the chance of becoming pregnant. Potential factors contributing to the increased prevalence are obesity, insulin resistance, and T2D.
Leptin will be an important player in this chapter. Here is a quick review slide from chapter one:

You Will Learn:
- Infertility and subfertility in men and women is rising.
- Underweight impairs fertility.
- Obesity impairs fertility in men and women.
- Growing adipose tissue disrupts sperm production.
- Growing adipose tissue disrupts the menstrual cycle.
- Low-grade inflammation reduces sperm, egg, and embryo quality.
- PCOS (polycystic ovary syndrome) is the leading cause for infertility in women.
Underweight Impairs Fertility
A successful pregnancy requires a lot from women. A pregnant woman needs to supply the energy and nutrients to grow her uterus, breast tissue, and the fetus, all while maintaining her own nutritional needs. The woman’s metabolism will profoundly change steering nutrients to those tissues and the fetus, sometimes at the cost of her own health.
For these reasons, it makes sense that the metabolism has a safeguard to ensure that women have the necessary nutritional resources for a successful pregnancy. If this is not the case and the woman is undernourished, hormonal regulations will kick in to prevent a pregnancy.
Secondary infertility refers to couples who have been able to get pregnant at least once but are unable to get pregnant again.
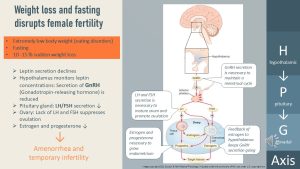
The key modulator of fertility is the hormone leptin, notifying other tissues of the adipose tissue status. If a woman has a very low BMI, or if the woman is losing weight rapidly, leptin secretion from the adipose tissue plummets. Reduced blood concentrations of leptin act on the hypothalamic-pituitary-gonadal (HPG) axis.
The HPG axis becomes active during puberty. In girls and women, cyclic hormonal secretion regulates female fertility. The main components of the HPG axis are:
- Hypothalamus: secretion of GnRH (gonadotropin-releasing hormone) which acts on the
- Pituitary gland: a small gland underneath the brain releasing LH (luteinizing hormone) and FSH (follicle stimulating hormones) in response to the GnRH.
- Ovary: LH and FSH have two main functions in the ovary.
- They stimulate the maturation and release of an egg.
- They also regulate the production of estrogen and progesterone by the follicle. This is relevant because estrogen and progesterone are necessary to grow the lining of the uterus—the endometrium (more about that in the pregnancy module).
- Estrogen is released into the blood circulation and gives feedback to the hypothalamus maintaining the HPG axis.
In normal-weight individuals, leptin keeps signaling sufficient energy stores to the hypothalamus which releases GnRH starting a menstrual cycle. In severely underweight women—for example women with eating disorders or athletes with RED-S—or after rapid weight loss, leptin blood levels are low. The leptin receptors in the hypothalamus register a lack of leptin as “a pregnancy would be unwise at this point.”
In low leptin blood concentrations the hypothalamus stops the secretion of GnRH. Without the GnRH stimulation, LH and FSH are not released from the pituitary gland. The ovaries do not receive the needed stimulation to mature and release an egg, and the endometrium does not grow. In consequence, the severely underweight woman will not ovulate (anovulation) at all or not regularly. She may not have a period (amenorrhea) and becomes temporarily infertile.
While amenorrhea or irregular periods should be discussed with a ObGyn, restoring some weight might be sufficient to restore fertility. This should be done by establishing healthy eating habits rather than eating high-calorie foods to increase weight rapidly. Even more importantly, establishing healthy, regular eating before pregnancy will help ensure a healthy pregnancy as well.
Fertility in underweight men is less researched. There is some evidence that sperm count is lower in underweight men, which might be connected to reduced fertility.
Obesity Impairs Fertility in Men and Women
As adipose tissue grows, leptin blood levels increase. The high leptin concentration should message to the HPG axis that nutrient stores are more than adequate and prompt the release GnRH. Why then do we see reduced fertility in people with obesity?
Disruption Of the HPG Axis in Men

In the fasting and underweight section, you looked at the HPG axis for women. The HPG axis works for men the same way, only the target gonads are the testes. GnRH secreted by the hypothalamus acts on the pituitary gland, which releases LH and FSH. FSH stimulates sperm growth while LH stimulates the Leydig cells to produce testosterone. Testosterone stimulates growth and maturation of sperm, but also feeds back to the hypothalamus to regulate the production of GnRH. Without testosterone, the hypothalamus will not produce GnRH.
Infertility due to obesity is much more complicated than infertility due to fasting or underweight. It is a multifactorial process and factors are adding up and interacting with each other. Studies from North Europe show that infertility in obese men is 36-53% higher than in normal weight men.
The testosterone-estrogen imbalance in obese men is directly correlated to the growing adipose tissue, but other factors impacting fertility such as leptin resistance, inflammation, insulin resistance depend more on the metabolic health of the men. While some men with obesity struggle with infertility, others become fathers without problems.
Androgens such as testosterone are needed to maintain the HPG axis and stimulate sperm production. Adipose tissue has an enzyme that converts androgens into estrogen. The more adipose tissue, the more androgens are converted and the more estrogen circulates in the bloodstream. This hormonal imbalance can disrupt the HPG axis and reduce fertility. Increased estrogen blood levels will also impact secondary sex characteristics.
Leptin: Growing adipose tissue causes an increase in leptin blood levels. As the adipose tissue keeps growing and leptin blood concentrations are chronically high, leptin resistance develops. This means that leptin will be less efficient in regulating the HPG axis. Less effective leptin means less secretion of GnRH, less LH and less FSH. Sperm and testosterone production are not stimulated sufficiently and decline.
Insulin resistance: Obesity combined with a genetic predisposition leads over time to insulin resistance. Insulin blood concentrations are permanently elevated in insulin resistance and pre-diabetes. High insulin blood concentrations will also disrupt the HPG axis.
Chronic inflammation: In the obesity chapter you learned that around 70-90% of people grow large adipocytes (hyperplasia) when adipose tissue increases. This genotype is connected to metabolic dysfunction. Overly large adipocytes, especially in visceral obesity, contribute to chronic systemic inflammation. Inflamed adipose tissue signals the inflammatory state to other tissues by secreting cytokines. Cytokines disrupt the HPG axis.
Sleep apnea is another concern for many obese people. Sleep fragmentation has been proposed as the mechanism by which sleep apnea disrupts nocturnal testosterone rhythm.
The mix and match of those factors can disrupt the HPG axis. Sperm and testosterone production declines leading to lower fertility. This condition is called secondary hypogonadism.
Reduced Sperm Quality
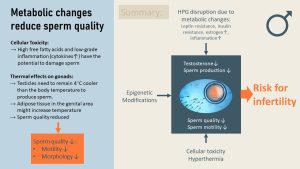
HPG axis disruption is only one issue when it comes to fertility in men with obesity. In addition to secondary hypogonadism, the quality of the sperm might be impacted as well. There are a couple of different reasons for this.
Oxidative cellular stress: In metabolically unhealthy men, blood concentrations of cytokines might be elevated due to low-grade chronic inflammation. This can lead to oxidative stress which impacts the production of sperm. Insufficiently managed pre-diabetes and T2D will lead to elevated glucose and fatty acid blood concentrations. High concentrations of both are damaging to cells, including sperm-producing cells in the testicles and sperm. Sperm anatomy and DNA is damaged.
Increased testicular temperature: In addition, growing adipose tissue around the gonads can insulate the testes resulting in increased temperature. The testes need to remain about 4 degrees cooler than the body temperature to produce sperm. Higher temperature in the testes can lead to less sperm production and low sperm quality.
Aside from measuring sperm concentration, the quality of sperm includes morphology (healthy structure and genome) and motility. Low-quality sperm is associated with lower fertility.
Sub-Fertility in Women
In women, obesity impacts both fertility and reproductive potential because even if an ovum is fertilized, the risk for the embryo not implanting or pregnancy loss is higher.

Ovulation and menstrual cycle: Leptin resistance, high insulin blood levels, increased estrogen and androgen blood concentrations disrupt the HPG axis in a similar way as discussed in men. Less GnRH secretion reduces LH and FSH production. The reduced blood levels of LH and FSH suppresses ovulation and a regular menstrual cycle.
Ovum quality: Low-grade inflammation and glucolipotoxicity due to increased glucose and free fatty acid blood levels in metabolically healthy women reduce the ovum quality by increasing oxidative cellular stress. Oxidative stress can damage the DNA. We know this because obese women undergoing fertility treatment might ovulate, but the resulting embryos are of lower quality, making survival less likely after implantation. What exactly does ovum quality mean? A damaged ovum has a reduced ability to be fertilized, and after fertilization to divide and mature into a healthy embryo. Part of the embryo will grow into the placenta. Low ovum quality will result in a placenta that is not able to nourish the fetus properly.
Endometrium Health: Adequate amounts of estrogen and progesterone stimulate the growth of a healthy endometrium (uterus lining) during the menstrual cycle. The disruption of the HPG axis leads to an endometrium that is less functional in supporting an early pregnancy.
Lower placenta and endomedrium function increase the risk for pregnancy loss.
PCOS Is the Leading Cause for Infertility in Women

So far, we have discussed how obesity-related metabolic changes lower fertility. Sub-fertility does not mean that women cannot get pregnant at all, just that it is not as easy. The main reason for infertility is polycystic ovary syndrome or PCOS
In the US, up to 20 % of women are affected by PCOS. 40 – 80 % of women with PCOS (depending on the study) are obese, and the majority of those women have fertility problems. Now, the question is whether the syndrome causes obesity or if obesity causes the syndrome. This is an important question that scientists have not answered fully yet. It looks like PCOS clusters in families, and this points toward a genetic component. This genetic disadvantage is exacerbated by environmental factors such as a poor diet, sedentary lifestyle, and weight gain. Environmental toxins such as endocrine disruptors are also discussed as aggravating the disorder.
PCOS is an endocrine disorder with a hormonal imbalance. Women with PCOS have increased androgen production, while estrogen and progesterone production are decreased. Visibly, this leads to women having unusual hair growth in the face (hirsutism) and acne. The hormonal imbalance also disrupts the HPG axis leading to irregular periods and cysts (fluid filled sacs) on the ovaries. Fertility is reduced.
Metabolically women with PCOS often have insulin resistance and low-grade chronic inflammation. Their gut microbiome tends to be less diverse with higher intestinal permeability. Since those women already have a predisposition for metabolic disease, their risk for T2D and CVD is increased.
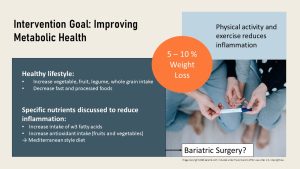
From studies, we know that a weight loss of 5 – 10 % will help many women with PCOS to restore fertility. Since those women tend to be insulin resistant, a low glycemic index diet is recommended along with regular low- to moderate-intensity exercise.
Since PCOS is currently an incurable disorder, the goal is to prevent weight gain and reduce weight by 5-10% if possible. Improving metabolic health will restore fertility in many women with PCOS. Research looking into nutrient supplementation, complementary medicines, and therapies, as well as specific diets—such as intermittent fasting, the Mediterranean diet, and keto diet—is low-quality and partially controversial. At this point, the main recommendation is to eat a plant-heavy, mixed, low-glycemic diet (low in sugar and refined flour products due to insulin resistance) and increase physical activity to at least 30 minutes of low- to moderate-intensity exercise.
Lifestyle Interventions to Increase Fertility
Keep in mind that when we, as nutrition professionals, talk about “weight” or “obesity” we mean metabolic health. By using these terms, we squeeze the entire adipose tissue chapter into those short words. The decline of metabolic health in women with obesity is due to a genetic predisposition and the aging process.
20-year-old women and men with obesity are often metabolically healthy and conceive without a problem. When the same individuals try to get pregnant in their 30s, they might have reached the threshold of metabolic health where fertility is impacted. Weight certainly plays a role in fertility, but there are additional controllable and uncontrollable factors that determine whether or not a couple can become pregnant.
What can couples do if getting pregnant is not as easy due to obesity? The first step to restoring fertility is starting a healthy lifestyle. An increased intake of fresh vegetables, fruits, legumes, and whole grains—as well as a decreased intake of fast and processed foods—should lead to a slow weight loss. Keep in mind that rapid weight loss will trigger a reduction of leptin blood levels and might reduce fertility in women for a while.
Increased aerobic activity supports dietary changes. Increased plant food intake and exercise will reduce chronic inflammation and improve sperm and oocyte quality.
The discussion of fertility-increasing foods is as old as humanity; however, scientific evidence only supports measures that help improve low-grade systemic inflammation and oxidative stress. Next to antioxidants from plant food and aerobic exercise, evidence supports an increased intake of omega-3 fatty acids from seafood and some nuts. Both strategies are included in the Mediterranean diet, which is sometimes recommended to improve fertility.
Interested In More Information?
Editors: Meryn Potts, Nicole Legler, Sydney Christensen
NUTR251 Contributors:
- Spring 2020: Justin Porreca, Kalen Codr, Dylan Fruhling, Bryce Heiser, Rose Davidson, Tim Gillespie
- Fall 2020: Bryson Krull, Clare Caraghar
Endocrine-disrupting chemicals (EDCs) are natural or human-made chemicals that may mimic, block, or interfere with the body’s hormones, which are part of the endocrine system. These chemicals are associated with a wide array of health issues (NIH).
Ovarian follicles are small sacs filled with fluid that are found inside a woman's ovaries. They secrete hormones estrogen and progesterone which coordinate the growth of the uterus lining and the menstrual cycle. Each has the potential to release an egg for fertilization.
Relative energy deficiency in sport


Feedback/Errata