1 Obesity Alters the Metabolism
Sabine Zempleni
Image copyright 2016 New York Times. Included under the provisions of fair use under U.S. copyright law.
In 2013 the American Medical Association declared obesity a complex, chronic disease requiring medical attention. The intention behind this move was to require physicians to start treating obesity and require insurances to pay for treatment and prevention. Hopefully, giving health care providers and insurances a nudge to treat obesity would reduce the prevalence of costly diseases such as type 2 diabetes and cardiovascular disease.
The backlash was instantaneous.
For starters the AMA House of Delegates overrode a recommendation made by the an AMA expert panel. In their statement the experts cautioned that a better diagnostic criterium for obesity would need to be developed given the limitations of the BMI. Despite the BMI shortcomings the expert panel still preferred to classify obesity a condition or disorder, but was overruled by the house of delegates.
While part of the medical community applauded the decision others cautioned that this move would have rather detrimental affects. Treatment of a disease tends to focus on pricey drugs, medical technology, surgical procedures and expensive clinic visits instead of addressing the root problems of the obesity pandemic. Given the millions of affected Americans some were concerned that the cost of treatment would be staggering.
Others pointed out that a disease designation would move the blame to the individual and would lead to increased weight discrimination. There was also concern that people would be released from personal responsibility and somebody else—the government, medical establishment—would be in charge.
Years later, the uproar has subsided, but different camps are still arguing about how to approach obesity. Physicians and other medical personal still haven’t learned how to address weight in the examination room and weight discrimination is rampant in the medical system. On the other hand some health care providers ignore the physiological facts (for example the “Healthy at Every Size” movement) and focus mostly on the psychological arguments. Disease designation or not, health care providers need to understand the connection between obesity and chronic diseases and need to be trained to address weight in a health care setting in a constructive way.
During this first module you will learn about the connection between obesity, systemic chronic inflammation (SCI) and chronic disease. Once you have a solid understanding of this interaction we will explore how lifestyle decisions at every stage of life can increase or decrease the risk for obesity and chronic diseases, and ultimately determine the long-term quality of life.
Before you dive into the next chapters you should review some terminology and basic facts regarding body composition. The following review slide summarizes facts about adipose tissue, BMI, the difference between the two, and how both are evaluated.

Two more concepts need review so you can fully understand the chapter:

There are three types of adipose tissue. White adipose tissue (WAT) looks white because it is build from adipocytes that are filled with a large storage bubble of triglycerides. The few mitochondria and nucleus are squished to the side of the cell. The size of the fat bubble can increase or shrink depending on the energy status. If the energy balance is positive triglycerides are stored in the adipocyte, if the energy balance is negative triglycerides are mobilized.
Brown adipose tissue (BAT) is located in specific areas of the body, for example between the shoulder blades (scapulas). Infants have large amounts of BAT to help maintain body temperature. Adults have little BAT left, but this can vary from person to person. BAT looks brown because it has small dispersed fat droplets and many mitochondria. In BAT the electron transport chain is uncoupled. This means that energy is produced in form of heat instead of ATP.
Beige adipose tissue resides within the white adipose tissue. Beige adipocytes look and function like white adipocytes until they are activated by cold-exposure or exercise. Under exposure an uncoupling protein (UPC1) is synthesized and the electron transport chain is producing heat instead of ATP. After the exposure the beige adipocytes can go back to being white adipocytes.
Later in this chapter and in the next chapter about type-2 diabetes it will be important to understand where the free fatty acids are coming from. When we are in a positive energy balance the surplus carbohydrates and amino acids are converted into triglycerides in the liver. Food triglycerides do not need to be converted. The surplus energy in form of triglycerides is transported to the adipose tissue. There are two transport routes. Triglycerides with long-chain fatty acids are transported by chylomicrons directly from the small intenstine to the adipose tissue. Secondly, the liver packs surplus triglycerides into VLDL (very low density lipoproteins). When chylomicrons and VLDL arrive at the adipose tissue the enzyme lipoprotein lipase located in the blood vessel wall splits triglycerides into fatty acids and glycerol. Fatty acids and glycerol diffuse into the adipocyte and are reassembled into triglycerides for storage.
If we are in a negative energy balance the adipose tissue releases glycerol and free fatty acids directly into the blood circulation. The fatty acids travel to the heart and skeletal muscles as well as the liver to undergo beta-oxidation. Glycerol travels to the liver to form glucose in the gluconeogenesis pathway.
You Will Learn:
1. Can you be healthy at every size?
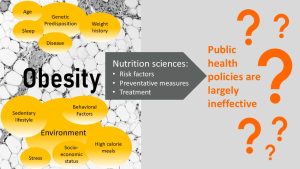
- Obesity is a problem for the US and the world.
- Weight gain and obesity are complex, multifactorial health conditions.
- Policies and interventions are frustratingly ineffective.
2. Adipose tissue is not just an energy store, but an endocrine organ.
- Leptin signals energy status to other organs.
- Adiponectin is involved in the regulation of glucose, fat metabolism and immune response.
- Pro-inflammatory cytokines signal inflammation status within the adipose tissue and between tissues and organs.
3. Obesity increases low-grade chronic inflammation (LGCI).
- Very large adipocytes leak fatty acids and are more likely to undergo cell death.
- Macrophages embedded in the adipose tissue trigger the innate immune system.
- Adipocytes become inflammatory amplified by increased leptin and decreased adiponectin.
- LGCI: Increased proinflammatory cytokines alter cell metabolism of other tissues.
4. Not all obese and overweight individuals are metabolically unhealthy.
- SCI and the altered cell metabolism fuel metabolic syndrome.
- Insulin resistance and slowly increasing fasting blood glucose levels are the main predictors for becoming metabolically unhealthy.
- Depending on genetics around 7 % of individuals with obesity are fully metabolically healthy.
Can You Be Healthy At Every Size?

At a very basic level obesity is defined as having too much body fat. The question is how much is too much? If you believe the media then people can be healthy at any weight. This statement is too simplistic though. While some overweight and obese people do not have any obvious health problems, we do know that obesity and to a lesser degree overweight increases the risk for non-communicable diseases such as cardiovascular disease (CVD), hypertension, type 2 diabetes (T2D), some cancers, non-alcoholic fatty liver disease, osteoarthritis or rheumatoid arthritis. Obesity also affects reproductive health and there is newer research showing that the risk for dementia is higher in people with obesity.
When we talk about obesity it is important to keep in mind that not everybody who is obese will develop those diseases. But, we need to separate personal risk and public health impact. Even if only a small fraction of the 70 % of Americans who are overweight and obese develop one or more non-communicable diseases it becomes a public health issue. Obesity is a major contributor to disability, medical costs, lost years of life and generates a huge societal cost.
The scientific community has researched factors contributing to obesity for a long time. Based on this initial evidence obesity was seen as a personal responsibility. Overeating and insufficient physical activity were cited as the main risk factors. During the last decades the research showed that this thinking was far too simplistic (see chapter 5).
Weight Gain and Obesity Are Complex, Multi-Factorial Health Conditions
Based on this extensive research we know that obesity is not just an issue of lacking will power. It became increasingly clear that weight gain and obesity are very complex multi-factorial health conditions. Multiple factors promote weight gain and make weight maintenance even after the weight is lost very difficult.
Genetic predisposition determines where body fat is stored and how much is stored. Some people have a more efficient metabolism and respond to temporary overeating by putting on weight (“thrifty metabolism”). During famines, which were common not too long ago, this genetic feature was a plus when it came to survival. Today, in an environment of constant abundance, a thrifty metabolism makes those people more prone for weight gain and ultimately chronic diseases.
Others have a genetic predisposition to store fat predominantly around the midsection. This is called central obesity. If the central adipose tissue is located around the organs inside the abdominal cavity it is called visceral obesity. Central obesity is more common in men and that is why it is also named android obesity. Gynoid obesity is more common in women with increased fat deposits around hips and thighs.
With age fat stores tend to increase and storage tends to shift toward central and visceral obesity in both genetic types. A genetic predisposition and advancing age do not necessarily mean that a person will become automatically obese. It will require a lot of work, knowledge and skills to maintain a healthy weight.
Eating a diet with a high energy density—high in added sugar, high in fat, low in fiber-rich plant food—makes it more likely that a person becomes overweight or obese. Research shows that people eating a plant-heavy diet, limiting fatty and sugary foods and reducing the consumption of ultra-processed foods maintain a healthy weight throughout life much easier.
Avoiding a sedentary lifestyle, increasing physical activity and regular exercise, support the nutrition side. While exercise, unless it is very extensive and intense, is often not sufficient to maintain a healthy weight, exercise and physical activity contribute to improved health and reduce the risk of non-communicable diseases at any weight.
The food environment impacts weight as well. In the US living a healthy lifestyle is an uphill battle at best and for underserved groups often not accessible. Availability of healthy foods at a reasonable prize, developing food preparation skills, and city planning that increases access to healthy foods and promotes physical activity would make it easier to choose healthy options.
Learned health behaviors such as how we choose food and how we eat our meals determine the trajectory of our weight.
Psychological factors such as a negative body perception, stress and emotional distress can be connected to emotional eating and lead to a steady weight increase.
Newer research also shows that other lifestyle choices promote slow weight gain. Lack of sleep might not be a main factor for weight gain but can contribute.
Based on this extensive knowledge health professionals developed health policies, recommendations, and intervention programs. Of course scientists researched the success of these programs and the results are rather disappointing. Health policies and intervention programs are frustratingly ineffective. Even if people lost a good amount of weight, the weight came back soon with a vengeance. Only 5 % of people losing weight will maintain the new lower weight long-term. Despite what the media says, that holds true for the popular keto and low-carb diets as well.
What is going on? Did those scientists miss anything?
Adipose Tissue Is Not Just an Energy Store But an Endocrine Organ
So far, we established that eat less, exercise more is too simplistic to explain why people fail to maintain a healthy weight. People gain weight over a lifetime as a combination of personal and environmental factors, and learned behaviors. The question is now why health interventions and nutrition policy are not able to turn the obesity epidemic around by addressing these factors.
You will start to understand why when you understand the function of the adipose tissue and the metabolic changes that happen progressively during weight gain.
Before the early 1990s we thought that white adipose tissue, or WAT for short, is composed from adipocytes and blood vessels. We thought that chylomicrons deposit part of the fat after a meal directly into the adipose tissue. In addition, very-low density lipoproteins (VLDL) transport triglycerides produced by the liver from surplus carbohydrates, amino acids or alcohol to the WAT.
There, lipoprotein lipase in the blood vessel wall is activated and hydrolyzes the triglycerides. The fatty acids can now pass into the adipocyte, the fat-storing cell, where they are rapidly re-esterified into triglycerides and stored. When energy is needed the triglycerides in the adipose tissue undergo lipolysis and fatty acids are released directly into the blood circulation and are taken up by cells in need for energy.
While this is still correct, our knowledge about adipose tissue has expanded immensely since then.
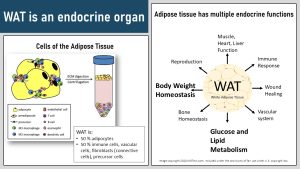
The figure on the left shows the cells of the adipose tissue. While adipocytes, the specialized cells storing triglycerides, account for most of the white adipose tissue volume, they make up only around 50 % of the cells. In addition to the fat storing adipocytes, adipose tissue also contains a plethora of other cells such as immune cells, fibroblasts stabilizing the adipose tissue, vascular cells connecting the adipose tissue to the blood circulation and precursor cells to grow new adipocytes.
Adipocytes do not just store fat but also communicate with other body tissues by synthesizing and secreting messenger molecules regulating blood pressure, triggering an immune system response, modifying glucose and lipid metabolism, regulating reproduction, maintaining bone homeostasis, regulation vascular growth and many more (infographic above, right).
Many of those messenger chemicals are also produced by other endocrine tissues and they collaborate with the adipose tissue to regulate the bodily functions mentioned above.
There is no way to address all messenger molecules released by the adipose tissue in this chapter. Instead we will focus on several molecules that are important to understand the connection between obesity and chronic diseases. You will study the basics for the adipokines leptin, adiponectin and resistin as well as the hormones estrogen and cortisol, and the proinflammatory cytokines.
Adipocytes Do Not Just Store Triglycerides But Communicate With Other Tissues

The adipose tissue also produces and secretes chemicals that are only secreted by the adipose tissue. These are called the adipokines. Adipokines are small peptides that are secreted into the vascular system and message the status of the adipose tissue to other tissues and organs. Targets include the brain, liver, pancreas, immune system, vasculature, muscle, and many other tissues. It is estimated that over 600 adipokines exist but they are by far not all researched. The best researched adipokines are leptin, adiponectin and to a lesser degree resistin.
Since this line of research is fairly new, we need to keep in mind that research is ongoing. While we lack understanding how those secreted chemicals work together, we do understand that expanding adipose tissue alters the secretion of those messenger molecules profoundly. As a consequence increasing fat mass changes how the metabolism works.
The adipokine leptin is a small protein that signals the energy availability of the adipose tissue to other organs. Primarily, leptin signals to the brain regulating satiety, but the same signals are also used to fine tune fertility, the inflammatory state of our metabolism and many more.
As adipose tissue grows—let’s say during the holiday season in November and December—more leptin is released into the blood stream. Receptors in the brain measure the increasing amount of leptin and signal fullness. The drive to eat, appetite, declines. When we work off those pesky pounds later in January leptin blood levels fall with shrinking adipose tissues and that increases our appetite.
If we don’t lose the weight and instead steadily increase adipose tissue, leptin blood levels keep increasing as well.
Once leptin blood levels are persistently high you would think that the body keeps signaling fullness, but now a counter regulation kicks in. The body becomes leptin resistant. This means since there is so much leptin in the blood the body produces less leptin receptors in the target organs. Leptin becomes now ineffective to signal the energy status and satiety.
In leptin resistance leptin blood levels will still decline during weight loss. The confused brain will read this as a starvation period and severe hunger will set in.
Adiponectin: While leptin secretion increases with growing adipose tissue, adiponectin secretion declines with growing adipose tissue. Adiponectin is less researched than leptin. So far we know that adiponectin is involved in the regulation of blood glucose levels, fatty acid breakdown and regulation of the immune response.
In lean people adiponectin circulates in high amounts and seems to have a protective effect against insulin resistance. The primary mechanisms by which adiponectin enhances insulin sensitivity appears to be through increased fatty acid oxidation and inhibition glucose production in the liver. The anti-inflammatory properties of adiponectin reduce atherosclerotic processes in the blood vessels.
Surprisingly, Obesity Alters the Secretion Of Other Hormones Important For Health
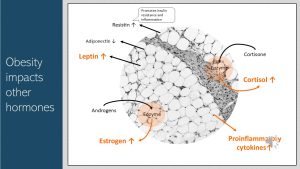
Cytokines—note that cytokines do not belong to the group of adipokines—are small messenger proteins and part of the cellular immune response. Cytokines can be proinflammatory (activating the immune response) or anti-inflammatory (dampening the immune response). Proinflammatory cytokines are secreted by many cell types to signal an inflammatory state. In obesity adipocytes are able to produce proinflammatory cytokines and the amount produced is proportional to the amount of adipocytes.
Cytokines attach to corresponding receptors of target cells and modulate the metabolism of the target cell. That way cytokines coordinate the immune response between cells in the same tissue, but also the immune response between cells of different tissues.This altered regulation has impact aside of the immune response. In insulin sensitive cells such as muscles increased amounts of cytokines decrease insulin sensitivity.
I would like to touch on two more secretory adipose tissue functions you will need to keep in mind for the fertility, type 2 diabetes and hypertension chapter.
Cortisol the main stress hormone in the body is involved in the regulation of glucose metabolism. Higher blood cortisol levels result in higher blood glucose levels. In a stressful situation more available glucose means more readily available energy. Originally, this mechanism helped the muscles react fast during the fight-or-flight response. Adipose tissue does not produce cortisol directly, but has an enzyme that converts the pre-cursor cortisone into cortisol. Therefore, more adipose tissue results in higher cortisol blood levels which in turn maintain higher blood glucose concentration.
Adipose tissue contains an enzyme called aromatase that converts androgens, the male sex hormones—women have also low amounts—into estrogen the main female sex hormone. In consequence, estrogen blood concentrations will rise with increasing adipose tissue in men and women. Keep this in the back of your mind until we talk about fertility and pregnancy.
RAS (renin–angiotensin system): We will get back to this later when we talk about obesity induced hypertension. Scientists think that adipocytes are able to secrete angiotensinogen one starting point of the blood pressure regulation. Adipose tissue also has high levels of the enzyme ACE. One of the function of ACE is to convert angiotensin I into angiotensin II. This mechanism can lead to increased blood pressure as adipose tissue growths.
How Does Ghrelin Fit In?
The hormone leptin is secreted by the adipose tissue signaling the energy status of the body. Ghrelin is the hunger hormone. When the stomach becomes empty and the last meal is a while ago, ghrelin is secreted into the blood circulation by the stomach. The circulating concentration of ghrelin is read by receptors in the brain and the feeling of hunger (growling stomach, weakness) sets in. As the stomach expands during while you eat your next meal ghrelin secretion decreases and blood concentrations fall. The feeling of hunger subsides and satiety sets in. Leptin on the other hand regulates the drive and motivation to eat, the appetite.
Obesity Increases Low-Grade Chronic Inflammation (LGCI)
Everybody talks about low-grade inflammation. Blogs and webpages warn of inflammation causing foods, recommend inflammation reducing diets, and peddle inflammation-reducing supplements. What is it?
Low-grade inflammation is a chronic stimulation of the innate immune response. A multitude of factors can trigger this process including having large amounts of adipose tissue, the aging process, an imbalance of gut microbiota, DNA damage and more. While inflammation due to infections or injuries can cause swelling, redness or an elevated temperature, low-grade inflammation will not show any typical inflammation symptoms. We can detect low-grade chronic inflammation by measuring proinflammatory cytokines circulating in the blood stream.
Quick Biology Review: What Is the Innate Immune System?
The innate immune system is the first line of defense against micro-organisms, foreign molecules, injury and cell death. The innate immune system is rapid, non-specific and non-anticipatory. The goal is to remove foreign or dead material—microbes or damage to cells and tissues—and communicate to the adaptive immune system.
In contrast, the adaptive immune system is specific and learns to anticipate and recognize foreign material. This type of immune response is slower and involves the binding of antigens and the secretion of immunoglobulins.
The innate immune system uses a range of inflammatory cells and proteins to detect threads, communicate those threads to body tissues and coordinate both immune systems.
One type of cells that will keep coming up are the macrophages. Macrophages are part of the innate/unspecific immune system and serve as a first defense. They derive from monocytes and have three broad functions:
• Identify foreign molecules or microbes and remove those via phagocytosis (envelop and destroy).
• Remove dead and dying cells in injured tissue via phagocytosis.
• Secrete cytokines and communicate that way with the adaptive immune system and other tissues.
Cytokines are a group of small signaling proteins coordinating the immune system and other cell functions. Examples of cytokines are interleukins and chemokines. Cytokines can activate the immune system (proinflammatory) and slow the immune system down (anti-inflammatory).
Balance is important: We tend to think about an immune response as a swift and hard intervention once the immune system is triggered but the opposite is true. Immune responses need to be measured and balanced because the processes will not just remove the virus or bacterium but damage tissue in the process. Think about the dreaded cytokine storm in COVID-19 patients or auto-immune diseases. The goal is to balance removal with as little damage to body tissues as possible.
Excessively Large Adipocytes Lead To an Inflamed Adipose Tissue
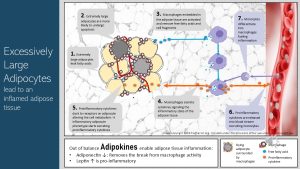
When white adipocytes becomes very large—above capacity—they will start leaking free fatty acids (FFAs) into the surrounding adipose tissue. At the same time super large adipocytes can become hypoxic and are more likely to undergo cell death (apoptosis).
Leaking fatty acids and dead cells shouldn’t be in a well functioning adipose tissue and this prompts an innate immune reaction: The macrophages, embedded in the adipose tissue, secrete pro-inflammatory cytokines into the interstitial space between adipocytes. The cytokines start diffusing in the adipose tissue and bind to receptors on the adipocyte surface. This signals an inflammatory state to other adipocytes.
This inflammatory signal changes adipocyte metabolism and turns adipocytes into what is called an inflammatory phenotype. This means that the adipocytes start to produce proinflammatory cytokines and other messenger molecules amplifying the signaling of the inflammatory status.
The proinflammatory cytokines in concert with other secreted messenger proteins increase inflammation in the adipose tissue. Amplified inflammatory signals will attract other immune cells such as monocytes from the blood stream. The monocytes can differentiate into macrophages and the newcomer macrophages start secreting cytokines as well. You get the point. The adipose tissue is now in a vicious inflammatory cycle.
There is another aggravating factor. In lean individuals the adipose tissue secretes smaller amounts of leptin and larger amounts of adiponectin. One function of adiponectin is to suppress macrophage activity in the adipose tissue and keep the innate immune system in balance. Declining adiponectin concentrations in the adipose tissue remove the break from the immune response.
Low-Grade Chronic Inflammation: Proinflammatory Cytokines Start Messaging To Other Tissues
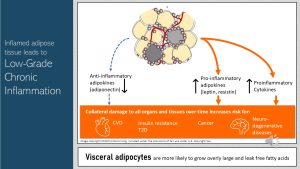
Expanding adipose tissue has the potential of becoming inflamed but the inflammation does not remain contained in the adipose tissue. Cytokines signal not only to cells in the same tissue to coordinate the immune response, but cytokines are also secreted into the blood stream and signal the inflammatory status of the adipose tissue to other tissues and organs. This chronically elevated secretion of cytokines is what we call systemic chronic inflammation or LGCI for short.
Proinflammatory cytokines coordinate the metabolism of many tissues and organs: Inflammation is not just a reaction of the immune system. Signaling molecules such as cytokines alter the entire metabolism profoundly for the short time the body is fighting the infection. Inflammation, as you all know from your last bout with flu or even soreness after an intense exercise session, triggers sickness behavior. Depending how severe the inflammation is, this will include sadness, anhedonia, fatigue, reduced libido, reduced food intake, altered sleep and social withdrawal. At a metabolic level inflammation increases insulin resistance and causes dyslipidemia. When you look at these metabolic and behavioral effects of inflammation it becomes clear that the purpose of those adjustments is to save energy and direct resources to the immune system. These reactions from other tissues ensure survival if life is threatened by a physical injury or a microbial threat. In LGCI the metabolic adjustments become permanent having a negative impact on health. The research area of chronic inflammation has only developed over the last decades. While we understand the big picture, specific mechanisms are discussed between scientists working in the field.
The circulating proinflammatory cytokines dock to receptors on the cell surface of other tissues. This changes the cell metabolism of those tissues as well. For example cytokines can dock to blood vessel epithelial cells triggering an inflammatory response or can dock to cells altering insulin sensitivity. Systemic, chronic inflammation contributes along with other risk factors to T2D, CVD, kidney disease and other chronic diseases. Recently evidence emerged that systemic inflammation might contribute to cognitive decline during aging and dementia as well.
Not every obese person develops chronic diseases, but evidence is strong that chronic inflammation triggered by an inflamed adipose tissue in obesity is the reason why non-communicable chronic diseases are much more common in obese individuals than in individuals in the normal and overweight range.
Emerging Science: EVs Enable Cells to Talk to Each Other
So far you learned two routes of communication between the adipose tissue and other cells and tissues: The adipose tissue secretes hormones and cytokines depending on the metabolic state of the adipose tissue. During the last years scientists discovered another important communication system between cells, tissues and organs: Extracellular vesicles or EVs for short.
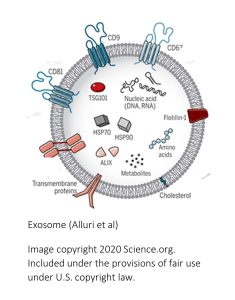 What are EVs? All cells release extracellular vesicles as part of their normal metabolism. EVs are tiny vesicles, a piece of membrane surrounding cargo (see image to the right) that is transported from cell to cell. If the cell membrane comes from the endosome the vesicles will be called exosomes (more about those specific EVs later.) The EV can also be pinched off from the cell membrane. The inside of the vesicle contains a cargo of regulatory proteins, lipids, DNA and RNA. EVs are secreted into the extracellular space and can either travel to other cells or specific target cells. Those target cells can be within the same tissue or the EVs are transported by the blood circulation to other tissues. Initially, scientists thought that EVs remove surplus cell material, but over the last decade it became increasingly clear that cells use EVs to communicate with each other. Depending on the metabolic situation cargo varies or the cell can regulate how many EVs and what type are secreted.
What are EVs? All cells release extracellular vesicles as part of their normal metabolism. EVs are tiny vesicles, a piece of membrane surrounding cargo (see image to the right) that is transported from cell to cell. If the cell membrane comes from the endosome the vesicles will be called exosomes (more about those specific EVs later.) The EV can also be pinched off from the cell membrane. The inside of the vesicle contains a cargo of regulatory proteins, lipids, DNA and RNA. EVs are secreted into the extracellular space and can either travel to other cells or specific target cells. Those target cells can be within the same tissue or the EVs are transported by the blood circulation to other tissues. Initially, scientists thought that EVs remove surplus cell material, but over the last decade it became increasingly clear that cells use EVs to communicate with each other. Depending on the metabolic situation cargo varies or the cell can regulate how many EVs and what type are secreted.
On a sidenote, proteins embedded in the EV membrane can direct the EV to a specific tissue or cell. A brand new area of research investigates how to use exosome shells to transport medication to specific cells in the body. For example, this would enable us to transport a chemo drug that wreaks havoc on all cells in the body only to the tumor leaving other cells unharmed.
What are the functions of EV? As you can imaging EVs have many functions. Studying nutrition sciences, the research focus tends to be on exosomes because they are involved in the coordination of the metabolism, interaction between muscle metabolism and health, coordination between gut microbiome and metabolism, inflammatory processes, development of insulin resistance, CVD, cognition, pregnancy, lactation to name a few.
You are what you eat? Exosomes are not just produced by human cells: As research evolved scientists made a couple of surprising findings that are important for nutritionists and dietitians. The first one is that exosome shells can withstand digestion. Since all cells produce exosomes, unprocessed food will contain exosomes as well. These exosomes can pass through our digestive system and act on our gut microbiome, but can also be absorbed into the blood circulation. In mice food exosomes were detected in the liver, spleen, and brain. In humans miRNA found only in milk exosomes, can be detected in the blood circulation when study subjects drink milk. This research is brand new, and the main question is how food exosomes impact cell metabolism or the gut microbiome. Secondly, our gut microbiome, modified by our food choices, also produces exosomes that end up in our blood circulation and have the potential to alter cell metabolism.
Not All Obese and Overweight Individuals Are Metabolically Unhealthy
As we have established, growing adipose tissue can fuel low-grade chronic inflammation which in turn alters cell metabolism. Over time chronic, metabolic diseases develop.
When scientist look at entire populations they find that many people with obesity are, as expected, metabolically unhealthy. The timeline when chronic diseases develop varies individually. While teenagers with severe obesity can develop type-2 diabetes or cardiovascular disease, others are obese but develop chronic diseases not until middle or high age. To make everything even more complicated some people with obesity are metabolically healthy, while some people in a lower weight range are metabolically unhealthy.
SCI and the Altered Cell Metabolism Fuel Metabolic Syndrome
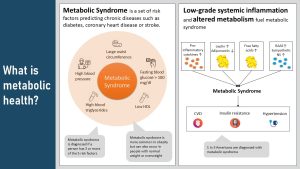
Metabolic syndrome is not a disease but a set of risk factors that predicts the risk for chronic diseases down the road. Five risk factors are considered:
- Large waist circumference: People with abdominal, especially visceral obesity have a high risk for developing chronic diseases.
- Hypertension: High blood pressure damages arteries. Constant damage to the endothelium promotes atherosclerosis which increases the risk for heart attack and stroke.
- High blood triglycerides: Hypertriglyceridemia is often a sign of health conditions increasing the risk for atherosclerosis. Excess triglycerides contribute to the the hardening and thickening of blood vessel walls.
- Low HDL: HDL is functioning like a little garbage truck circulating the blood vessels and picking up surplus cholesterol or cholesterol from dead cells. HDL then transports the choletsrol back to the liver for recycling. High HDL helps keep blood vessels healthy, lowering the risk for heart attack and stroke.
- Fasting blood glucose over 100 mg/dl indicating prediabetes: High blood glucose also damages blood vessels contributing to atherosclerosis and organ damage (note that insulin resistance is not a criterium).
If a person is diagnosed with 3 or more of those criteria, the person is diagnosed with metabolic syndrome. A diagnosis means that the person is a higher risk for type-2 diabetes, heart disease and stroke.
Low-grade chronic inflammation is fueling metabolic syndrome. In addition to chronic inflammation, the imbalance of leptin and adiponectin, increased release of free fatty acids and the activation of RAS (see hypertension chapter) contribute to metabolic syndrome as well.
Metabolically Healthy and Unhealthy Obesity Is Determined By Genetics and Modulated By Environmental Factors
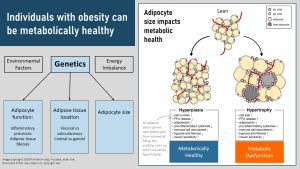
The main factor determining if a person with obesity is metabolically healthy or not is determined by genetics. Genetics determines how the adipose tissue grows, where it is located and how large adipocytes can become. Environmental factors such as food environment, physical activity, stress, sleep and the degree of the energy imbalance are modulating factors.
Specifically, some people have a genotype that increases adipose tissue by generating more and smaller adipocytes. This is called hyperplasia and is less connected to systemic chronic inflammation.
Other people’s adipose tissue fills up existing adipocytes to capacity producing very large adipocytes. This is called hypertrophy. Overly large adipocytes are connected to the development of low-grade inflammation.
The location of the adipose tissue is important as well. Visceral obesity—excessive adipose tissue located within the body cavity—fuels chronic inflammation more than subcutaneous adipose tissue. Even some lean individuals have excessive amounts of visceral fat and therefore develop LGCI.
As people reach middle age the adipose tissue location starts to change. Hormonal changes in middle age promote central and visceral obesity. Details are discussed in the aging chapter.
In summary the current evidence points toward overly large adipocytes and visceral obesity as the main predictors for LGCI. But, does this mean that people with this type of obesity automatically develop CVD or T2D early in life and die early?
Lifestyle plays a major role as well. Eating a diet rich in fruits and vegetables and having an active life style promote anti-inflammatory mechanisms and curb LGCI. As a consequence metabolic disease progresses more slowly even if a genetic predisposition exists. Structured exercise for health seems to stimulate inflammatory processes as muscle tissue is broken down and repaired (sore muscles) which in turn damps down LGCI.
Together, genetics and environmental factors explain partially why some overweight and obese people stay metabolically healthy for a long time while others develop T2D already in their teens. Keep in mind that the transition between those metabolic types is fluent.
Emerging science: What is the role of brown and beige adipose tissue in metabolic health?
A new line of research is looking into beiging of white adipose tissue to enhance metabolic health. This reasearch is promising but at a very early stage. Scientists are working to understande why some people have more beige adipose tissue, what triggers beiging and how the amount of beige adipose tissue impacts metabolism.
Brown adipose tissue grows in the fetus during the last months of pregnancy in preparation for the life in the cold world. As the infant grows into a child much of the brown adipose tissue disappears. Adults have still some areas with brown adipose tissue left. How much seems to be genetically determined.
Beige adipose tissue growths after birth and then becomes dormant (turning white) until cold exposure stimulates the beiging. Beiging means that the adipocytes increase the number of mitochondia and the cell starts burning energy to produce heat. The image below shows the beiging during a cold exposure trigger. The dark areas are the beige adipose tissue.
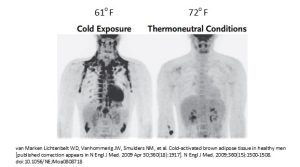
Another trigger of beiging is exercise. During inactivity and neutral temperatures the beige cells reduce the number of mitochondia and become white adipocytes again. Just as white adipose tissue, beige adipocytes send signals to the metabolism. The new research shows that people with low amounts of beige adipose tissue also tend to have more visceral fat, tend to have a higher weight and lower insulin sensitivity, and have more metabolic diseases. Before you turn down the thermostate or jump into an ice bath, keep in mind that we do not know why people have more beige fat. This is super-interesting early research.
Last Question: How Many People With Obesity Are Metabolically Healthy?
When you go online different numbers are floating around. Some sources state 10 – 30 % are metabolically healthy others estimate 50 %. A review study looked at all the existing studies and came to the conclusion: It depends how you define metabolically healthy.
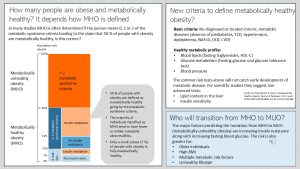
Lets first define what metabolically unhealthy obesity (MUO) is: Obesity that is paired with low-grade chronic inflammation and manifested metabolic dysfunction. The normal regulation of metabolic function is pretty much hijacked by the hormones and proinflammatory cytokines secreted by the adipose tissue.
The definition of metabolically healthy obesity (MHO) is trickier because MHO is not defined consistently. In many studies metabolically healthy obesity is diagnosed if the study participants have two or less metabolic syndrome criteria. This is the reason why you read sometimes that 50% of people with obesity are metabolically healthy. But, are they healthy? A review summarizing all existing studies questioned this definition.
When the scientists looked at the 50 % (supposedly) metabolically healthy people with obesity the majority of those individuals were not completely healthy but had only fewer and milder metabolic abnormalities. For example, using the commonly used metabolic syndrome criteria people can have elevated blood glucose or insulin resistance (not a metabolic syndrome criterium) and still be classified as MHO.
Insulin resistance is a strong predictors for declining metabolic health with age. When the scientists used a new, more restrictive definition only a small subset of people with obesity, around 7 %, was fully metabolically healthy. The table above also summarizes the new proposed criteria to define MHO. If you are interested in the entire study: Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest. 2019;129(10):3978-3989. doi:10.1172/JCI129186.
The question is then who will transition from metabolically healthy to unhealthy obesity. As people age and have an unhealthy lifestyle chances are that their metabolic health will slowly decline. If people have a high BMI and are developing multiple risk factors, the risk for being diagnosed with a chronic disease increases. The main predictors for MUO though are insulin resistance along with increasing blood glucose.
In conclusion, growing adipose tissue comes with profound metabolic changes. Hunger and satiety regulation becomes progressively inefficient, hormonal changes affect the metabolism, and systemic chronic inflammation alters the immune response and affects other tissues. Our understanding of these complex processes is still limited to broad ideas. Today, research works on understanding the mechanisms that connect growing adipose tissue to chronic diseases and most importantly the reversal of those metabolic changes.
Interested? Want to Know More?
Obesity is a major source of systemic chronic inflammation but other factors contribute as well. Here is a Nature article reviewing what we know about the intersection of systemic chronic inflammation and the development of chronic diseases:
If you are as fascinated by exosomes as I am, here is a great review article going into more detail:
American Medical Association
Andro- means male (Greek)
Splits triglycerides into glycerol and fatty acids
Attaching three fatty acids to one molecule of glycerol
Having too little oxygen to survive
The inability to feel pleasure
Increased lipid and cholesterol blood concentrations
Endosomes are intracellular sorting organelles. They sort molecules and regulate traffic of molecules in the cells. This can include directing molecules to the correct cellular compartment, marking molecules for recycling or packaging cargo into exosomes.
Space outside the plasma membrane of a cell. The extracellular space includes the interstitial space (between cells) and the intravascular space (blood vessel system, lymphatic system.)
EV secreted by the endosome
Lining of the blood vessel
Metabolically healthy obesity


Feedback/Errata