11 The Woman Changes: Anatomical, Physiological and Metabolic Adaptations During Pregnancy
Sabine Zempleni
You have probably seen excited social media posts of positive pregnancy tests or ultrasound results announcing that a couple is expecting a child. Around week six counting after ovulation, cardiac cells have grown into two tubes. At this point, electric activity of those cells can be detected using a vaginal ultrasound. For many parents, this rhythmic blip on the ultrasound is the first exciting sign that the tiny embryo (measuring 4 to 5 mm) is there. While the embryo stays tiny for a while longer, the woman‘s body is going through major physiological and metabolic changes to prepare for the accelerating growth of placenta and fetus that will start around week 16.
During this first pregnancy chapter, you will explore this remodeling of the woman’s body.
Before you learn about the hormonal and physiological changes during pregnancy, refresh your biology knowledge and learn pregnancy terminology:
You Will Learn:
- Pregnancy hormones drive anatomical and physiological adaptations:
- While the placenta is established during the first ten weeks, embryonal growth depends on the yolk sac and histiotrophic nutrition. The placenta is connected to the maternal blood circulation around week 10-12.
- Cardiorespiratory system: Progressively expanding capacity enables placental and fetal growth.
- Renal system: Progressively expanding capacity removes metabolic waste fast.
- Maternal anatomical and physiological adaptations start early in pregnancy and build on each other to prepare for hemotrophic nutrition at week 10-12 supporting rapid fetal growth after week 16.
- Physiological adaptations do not just support growth of fetus and placenta but also prepare for delivery and lactation.
- Metabolic adaptations during the second and third trimester of pregnancy direct nutrients toward the fetus:
- Physiological insulin resistance will develop during the second and third trimester of pregnancy.
- Fasting during pregnancy is not recommended.
- An atherosclerotic blood lipid profile develops during pregnancy
Pregnancy Hormones Drive a Progressively Increasing Cardiorespiratory and Renal Capacity
The Endocrine System
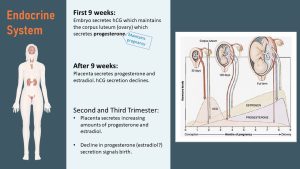
Once the blastocyst has implanted, it starts secreting hCG (human Chorionic Gonadotropin) which signals to the woman’s endocrine system that fertilization and implantation has successfully taken place.
Without the hCG signal, the corpus luteum in the ovary would die down and the menstrual cycle would end with the shedding of the endometrium. When hCG is secreted into the maternal circulation, the corpus luteum is maintained and keeps secreting progesterone during the next nine weeks. The interaction between hCG and progesterone from the corpus luteum maintains the pregnancy during the first months.
Coincidentally, hCG is the hormone that is detected in over-the-counter pregnancy tests.
After nine weeks, the placenta has grown sufficiently to take over the secretion of progesterone and estradiol. This causes the hCG secretion by the placenta to decline. The corpus luteum is not needed anymore to maintain the pregnancy and dies down.
During the second and third trimester, the levels of progesterone and estradiol keep increasing all the way up to birth. A drop in progesterone ends the pregnancy and starts the process of delivery. Estradiol levels are also rapidly declining, but it is unclear how estradiol is involved in delivery.
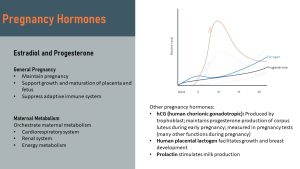
The main function of the two major pregnancy hormones estradiol and progesterone is well known: Maintain the pregnancy and support growth and maturation of placenta and fetus. The effects of those two hormones reach much farther though. They orchestrate the adaptations of the maternal metabolism by adapting blood circulation, energy metabolism, waste excretion, and bone metabolism to the needs of the growing placenta and fetus.
Estradiol—the form of estrogen produced by the placenta—maintains the pregnancy by regulating progesterone, stimulating maturation of fetal organs, increasing the uterine blood flow, stimulating the uterus growth, preparing the breast for lactation, and modulating the renin-angiotensin system.
Progesterone helps establish the placenta, calms the smooth muscles of the uterus to avoid uterus contractions, and relaxes blood vessels and gastrointestinal system.
Both hormones also work together to suppress the immune system. Estradiol and progesterone shift the innate and adaptive immune responses from an inflammatory to an anti-inflammatory phenotype to prevent rejection of the fetus—which is considered foreign genetic material—by the maternal immune system.
Estradiol and progesterone might be the major pregnancy hormones, but there are others. For example, lactogen facilitates growth and breast development, and prolactin stimulates milk production.
The Cardiorespiratory System: Progressively Expanding Capacity Enables Placental and Fetal Growth
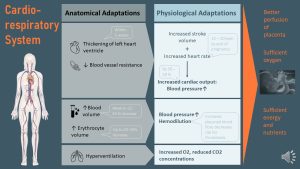
Within the first five weeks of the pregnancy, increasing estradiol and progesterone blood concentrations trigger major cardiovascular system adaptations. The purpose of this cardiovascular remodeling is to maintain good perfusion of the placenta and to provide sufficient oxygen and nutrients to the fetus and placenta.
Cardiac output increases: The heart muscle thickens, resulting in an increased cardiac stroke volume. In addition, the heart rate increases slightly. The relaxing of the blood vessels lowers the blood pressure initially. As you know from chapter 4 hypertension this will trigger the sympathetic nervous system raising heart rate and stroke volume. Together, these adaptations increase the cardiac output. By the end of the pregnancy, the cardiac output is 30-60% higher than before the pregnancy, and blood pressure is slightly elevated as well.
Blood volume increases: Blood vessel resistance decreases, causing lowered blood pressure in the first trimester. A lower blood pressure—think back to the hypertension chapter—will trigger the RAAS system, which will increase the blood pressure back to normal by retaining more water and sodium. Between weeks 6-32, the blood volume will increase up to 45%.
Both changes accelerate over the course of the pregnancy to perfuse a growing placenta and deliver increasing amounts of nutrients and oxygen to the fetus.
Erythrocyte volume increases: Once the fetus’ growth takes off, larger amounts of nutrients and oxygen are needed. A 20-30% increase of erythrocytes will help perfuse the placenta with enough oxygen.
Hemodilution takes place: While the erythrocyte volume increases by only 20-30% over the course of the pregnancy, the blood volume increases by around 45%. More fluid leads to hemodilution. When a hemoglobin test is done at a prenatal checkup, less erythrocytes per unit plasma might look like anemia. This makes it hard to distinguish between true anemia and pregnancy related relative anemia. The purpose of hemodilution is to possibly prevent thrombosis during pregnancy.
Hyperventilation: Breathing rate becomes faster during pregnancy because the growing fetus and placenta will require more oxygen. Breathing is adjusted so arterial oxygen levels are maintained and CO2 blood levels remain low.
Overall, the adaptations ensure optimal perfusion of the placenta supplying oxygen and nutrients, but also removing waste rapidly.
Renal System: Progressively Expanding Capacity Removes Metabolic Waste Fast

At the beginning of the pregnancy, the kidneys undergo anatomical adaptations. They increase in size, volume and weight. Renal blood flow increases 60-80% and the filtration of waste increases by 50%.
The purpose is to increase metabolic waste removal. Once the fetus starts growing rapidly, both fetus and placenta produce additional metabolic waste products, which requires the kidneys to work harder.
As already pointed out under cardiorespiratory changes, blood volume increases. One mechanism responsible is the relaxation of the blood vessels, but pregnancy hormones also trigger an increased secretion of aldosterone. Aldosterone acts on the kidneys to increase water and sodium retention and potassium excretion, contributing to an increased plasma volume.
The Placenta: Development Starts at Implantation

The placenta is defined as “the fusion of fetal membranes with the uterine mucosa for the purpose of maternofetal exchange of nutrients, gases, and waste substances.” Placental development starts at the time of implantation, as soon as the blastocyst invades the endometrium of the uterus.
At this stage, the blastocyst consists of an outer layer of cells covering a vesicle. This layer is called the trophoblast (see image above on the top right). Inside the vesicle is an inner cell mass, called the embryoblast. The placenta is mainly derived from the trophoblast, whereas the embryoblast develops mostly into the umbilical cord and the fetus.
Immediately after implantation, cells from the trophoblast start to grow rapidly, infiltrating the endometrium. Small spaces, the vacuoles, form and grow into several cavities called intervillous space (IVS) or lacunae.
Initially endometrial glands of the uterus, then maternal veins and lymph vessels are invaded by trophoblast cells and connected to the IVS. As soon as this connection takes place, the glands can start to secrete fluid and nutrients into the IVS. The nutrients are taken up by the yolk sac via phagocytosis to nourish the embryo.
For ten weeks, the embryo is nourished by the yolk sac (see infographic above) by taking up secretions from the endometrial glands. The gland secretions seem to contain glycogen particles and glycoproteins. Feeding by glands is called histotrophic nutrition. During the first trimester, this type of nutrition is sufficient for the tiny embryo as it focuses on cell differentiation and organ development, and not on increasing cell mass.
At the same time, fetal blood vessels start forming, and the fetal side of the placenta starts growing villi into the IVS in order to increase the surface for nutrient exchange.
On the maternal side, the spiral arteries in the uterus are invaded and remodeled by fetal cells and connected to the IVS, which is now one large cavity (lacuna). Once this is accomplished at the end of the first trimester, the placenta becomes functional and the nutrition of the fetus becomes hemotrophic (by blood). The IVS is now flooded with blood.
At this point, the fetus and placenta are still small. The placenta will keep developing and growing proportionally to the fetus to provide sufficient nutrients and oxygen until the end of the pregnancy.
Maternal Anatomical and Physiological Adaptations Build Upon Each Other to Prepare for Rapid Fetal Growth
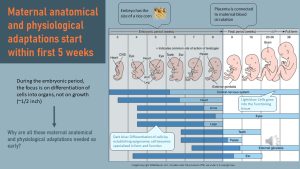
Weeks 3-8 of a pregnancy are called the embryonic period. The embryo is tiny, and development focuses on differentiating cells into structures that later become the different organs and tissues. Differentiation of cells takes place during the first sensitive period; the epigenome is reestablished and switches genes off and on to allow for the establishing of different organs and tissues (see epigenetics chapter).
At this point, the placenta is still small and developing. Before weeks 10-12, the placenta is not connected to the mother’s blood vessel system and the embryo receives nourishment from the yolk sack as well as histiotrophic nutrition from the uterus gland secretions. This stage of development does not require much energy and nutrients. Why does the mother then undergo such profound anatomical and physiological adaptations so early in the pregnancy?
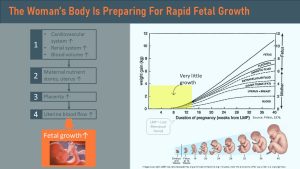
The early increases in metabolic function (cardiovascular system and renal system capacity increases) are preparing the mother for the huge demands on her body that will occur later in pregnancy when the fetus and the placenta start growing at a rapid rate.
During those early weeks, the mother will accumulate adipose tissue and other nutrient stores, develop an enlarged heart and kidneys, expand blood volume, and grow her uterus.
Once the uterus is ready, the connecting of the fetal side to the maternal blood circulation can take place. The delivery of nutrients via blood circulation allows for larger amounts of nutrients to be transferred to the placenta—allowing placental growth to take off. Consequently, placental diffusion with blood increases, which leads to an increased transfer of nutrients to the fetus.
Finally, around week 16, fetal growth can take off. All the maternal metabolic adaptations build on each other in an elegant way to support the growth and development of a healthy fetus.
Maternal Anatomical and Physiological Adaptations Serve Three Purposes: Fetal Growth, Preparation for Birth, Preparation for Lactation
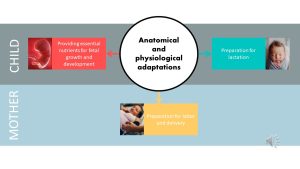
So far, I talked about the maternal adaptations and how they support the development and growth of the fetus, but those changes have two more purposes.
Preparation for birth: hormones increase, especially during late pregnancy, preparing the mother for birth. Muscles and tendons are relaxed and loosened so the fetus has enough space to pass through the narrow birth canal. Increased nutrient and immunoglobulin transfer prepares the fetus for life outside the uterus.
Preparation for breastfeeding: Breast growth and maturation takes place very early in the pregnancy. In fact, by mid-pregnancy the woman is, in principle, ready to nurse an infant. High progesterone blood levels prevent milk production in this state.
Metabolic Adaptations Direct Nutrients to the Fetus
During the first trimester the metabolic goal was to store energy in order to get ready for rapid placental and fetal growth. Insulin sensitivity increased slighly during those initial weeks of the pregnancy leading to more glycogen production and increase of adipose tissue. The metabolic goal changes as the growth of fetus and placenta accelerates. The main metabolic goal during the second and third trimester is to supply the fetus with a constant flow of glucose, nutrients and oxygen while removing waste products rapidly. You already learned about the anatomical and physiological adptions supporting this goal. Next you will learn about the metabolic adaptions.
Physiological Insulin Resistance Will Develop During the Second and Third Semester of Pregnancy
So far you learned about pathological insulin resistance during obesity and the development of T2D. Metabolic changes associated with expanding adipose tissue increase the amount of circulating cytokines which disrupt signaling between the insulin receptor and the glucose transporter in insulin sensitive tissue (see T2D chapter).
This needs to be distinguished from physiological insulin resistance, or lower insulin sensitivity during pregnancy.
During mid pregnancy, the metabolism starts adjusting to direct glucose to the growing fetus while the mother is increasingly relying on fat as an energy source. This shift is driven by a physiological decrease in insulin sensitivity.

The physiological insulin resistance affects the maternal muscle which takes up less glucose. Similar to pathological insulin resistance, this will keep blood glucose concentrations elevated longer after a meal, allowing the placenta to direct more glucose to the fetus.
Higher blood glucose levels will trigger more insulin secretion by the maternal pancreas, allowing the blood glucose levels to normalize between meals.
The insulin resistance of the adipose tissue—insulin suppresses lipolysis and insulin resistance eases this metabolic break—will lead to increased lipolysis and release of more free fatty acids into the circulation. During physiological insulin resistance this is not a problem. The mother will use the free fatty acids as a preferred energy source. Long-chain fatty acids are also directed to the fetus as a building block for fetal tissues.
Keep this interesting metabolic change in mind to understand why obesity is a risk factor for developing gestational diabetes in the weight and pregnancy chapter.
Fasting During Pregnancy Is Not Recommended
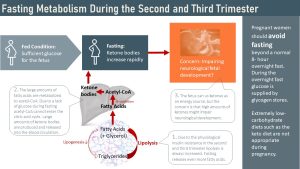
The fetus needs a constant supply of glucose as an energy source. While the fetus is able to use ketone bodies (not fat though) as an energy source, exposure of the fetus to high ketone concentration seems to have a negative impact on neurological development. Therefore, maternal fasting is not recommended.
The key to understanding the reasoning behind this recommendation is that the insulin resistant adipose tissue undergoes physiologically increased lipolysis already since the end of the second trimester.
When eating a healthy balanced diet, which includes three meals and maybe a couple of healthy snacks a day, the mother’s metabolism will direct glucose to the fetus and placenta, and will use fat as the preferred energy source. In this situation, ketone blood concentrations are normal and low.
Let’s say the mother has her last meal at 6:30 pm, undergoes a normal overnight fast, and the skips breakfast the next morning. At this point glycogen stores are depleted and insulin blood levels low. The adipose tissue already has a higher rate of lipolysis, but now the insulin break on lipolysis is completely removed. Consequently, the adipose tissue is now flooding the circulation with fatty acids. Free fatty acids undergo beta-oxidation and yield large amounts of acetyl-CoA.
Here is the problem. Since glucose is missing to form oxaloacetate, the entry intermediate of the citric acid cycle, the large quantities of acetyl-CoA cannot enter the citric acid cycle and build up in the cell. The cell resolves this by forming ketone bodies and releases those into the bloodstream.
This means that the fetus is forced to use ketone bodies as an energy source as well and will experience high ketone blood concentrations.
We know that the fetus develops best by having a constant supply of glucose. While the fetus is able to use ketone bodies as an energy source, animal studies have shown that high amounts of ketone bodies impact the neurological development of a fetus. Naturally, research in human fetuses is unethical so we are not 100% sure about the situation in human fetuses. Due to the precautionary principle—better safe than sorry—pregnant women should avoid meal skipping and fasting longer than a period of 12 hours. The diet should also supply a continuous glucose supply.
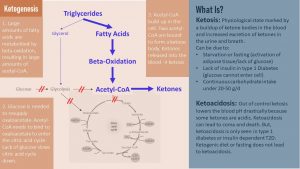
If you have forgotten how ketosis works here is a review infographic:
The Woman Develops an Atherosclerotic Blood Lipid Profile During Pregnancy
During the last two trimesters the pregnant woman will progressively develop an atherosclerotic blood lipid profile.
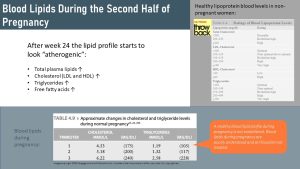
The throw back table lists ratings of blood lipid levels in non-pregnant adults. Compare those ratings to the approximate changes during advanced pregnancy in the green table.
During the third trimester women have cholesterol and triglyceride levels that would be rated as high. While poorly understood, this is thought to be physiological. After birth the blood lipids will usually return back to normal.
Since we know so little about blood lipids during pregnancy there are no established ratings for the blood lipid profile in pregnancy and elevated levels are normally not treated.
Overall, it becomes clear that the body of a pregnant woman does not only increase in size by grow an uterus and fetus. Directed by the pregnancy hormones the woman’s body undergoes profound anatomical and metabolic changes. The next chapter will connect those changes of the maternal body to the nourishing of the fetus.
Editors: Meryn Potts, Sydney Christensen, Gabi Ziegler
NUTR251 Contributors:
- Spring 2020: Brett Freitag, Jenna Junker, Danae McKenzie, Rawan AL Jabri, Caroline Leibel, Terrell Horton, Grant Young
- Fall 2020: Ken Zhao, Morgan McCain, Miles Judson, Bryson Krull, Kaitlyn Higgins, Clare Caraghar, Megan Appelt
While I chose to use the term pregnant woman in this chapter keep in mind that transgender men who retain their uterus can become pregnant as well. If you are interested in this topic here is a systematic review exploring the needs of transgender men during pregnancy and lactation: MacLean LR. Preconception, Pregnancy, Birthing, and Lactation Needs of Transgender Men. Nurs Womens Health. 2021;25(2):129-138. doi:10.1016/j.nwh.2021.01.006
A group of cells inside the ovaries formed from the cells surrounding the oocyte. The corpus luteum appears during after ovulation and secretes progesterone to maintain the endometrium. If fertilization does not take place and an embryo is not implanted the corpus luteum breaks down progesterone secretion ceases and the endometrium is shedded during menstruation.
The volume of blood pumped out of the left ventricle of the heart during each contraction.
Red blood cells
uterus lining
Intervillous space
The yolk sac is a small membranous structure outside the embryo with various functions during early embryonic evelopment. One of the functions is nourishing the embryo.
Fingerlike protrusions similar to the ones in the small intestine.



Feedback/Errata
20 Responses to The Woman Changes: Anatomical, Physiological and Metabolic Adaptations During Pregnancy