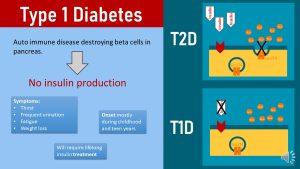
3 Type 2 Diabetes Is a Complex Chronic Disease
Sabine Zempleni



You will learn:
- Type 2 diabetes (T2D) is not a disease of the affluent and aging anymore:
- T2D diagnosis is on the rise worldwide especially in low- and middle income countries and younger population groups.
- Health disparity: In the US Native Americans, Black Americans and Hispanic Americans are most affected.
- T2D develops as a consequence of obesity, lifestyle and genetic predisposition.
- T2D develops over years to decades:
- Chronic systemic inflammation disrupts insulin signaling and damages beta-cells
- T2D development is complex and highly individual
- T2D is the interaction between insulin resistance and an inadequate insulin production by the beta cells of the pancreas.
- T2D development: Insulin resistance → impaired glucose tolerance/pre-diabetes → reversible T2D → irreversible T2D.
- Age at onset of T2D and progression varies individually. This is determined by a combination of lifestyle, genetic predisposition and age.
- Intervention often starts when damage is already done:
- T2D complications are dreaded consequences of glucose and free fatty acid toxicity affecting blood vessels.
- Macro- and microvascular damage lead to nerve damage, slow wound healing, diabetic foot, amputation, kidney failure, blindness and increase a risk for CVD.
- Pre-diabetes and early T2D are silent diseases without symptoms.
- Early metabolic changes cannot be detected efficiently by routine lab tests. Diagnostic tests tend to detect changes in glucose metabolism when the fasting blood glucose concentrations are elevated.
- Lifestyle interventions for pre-diabetes and T2D include a plant-heavy eating pattern, an active lifestyle and an exercise program:
- Dietary interventions have the goal to improve insulin resistance, blood glucose management and prevention of CVD.
- There is no ideal diet for the management of T2D, but several diets have shown benefits. Those diets have as hared pattern of high nutrient density, low energy density and high fruit, vegetable, legume and fish intake in common. Intake of added sugar and saturated fat is low. The preferred macronutrient distribution is controversial.
- Physical activity intervention has three goals: Improved/reversed insulin resistance, blood glucose management, and CVD prevention.
- Exercise recommendation: 30 – 60 minutes of combined strength training and moderate intensity aerobic exercise on at least 4 days of the week. Increased overall physical activity.
- If lifestyle interventions fail to induce weight loss of 5 – 10 % and fail to improve metabolic health, bariatric surgery might be considered.
T2D Is Not a Diseases of the Affluent and Aging Anymore
T2D was for a long time predominantly found in older people from affluent countries in Europe and North America and connected to an abundance of food. This is the reason why we still think about T2D as a chronic disease triggered by affluence.
Today, T2D has become a global pandemic and prevalence is rising steeply in low- and middle-income countries. In high income countries T2D is found disproportionally in lower income population groups. The global prevalence of diabetes—all types but T2D represents the majority of cases—among adults over 18 years rose from 4.7% in 1980 to 8.5% in 2014. If you consider a world population of 7.7 billion people this is a staggering amount of diabetes cases.
Diabetes is a major cause of blindness, kidney failure, heart attacks, stroke and lower limb amputation. Those complications can lead to premature death. The WHO estimates that diabetes was the seventh leading cause of death worldwide.
T2D Prevalence is on The Rise in the USA
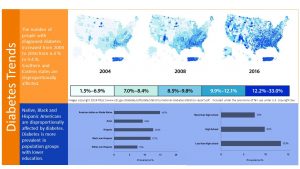
In 2018 an estimated 10 % of the US population older than 18 years had been diagnosed with diabetes. In addition it is estimated that another 3 % of the population is not aware that they meet criteria for a diabetes diagnosis. Most likely this is due to a lack of access to health care. T2D is a silent disease and symptoms such as severe fatigue only become apparent after years when T2D progresses. Keep in mind that this prevalence includes other forms of diabetes as well, but the majority of the cases are T2D.
Combined, this means a staggering 34 million Americans had diabetes in 2018.
When we look only at older populations it gets even worse. The prevalence of diabetes increases with age and reaches a prevalence of 14.5 % in middle aged and older populations.
T2D Prevalence Varies By Race And Education
There is a clear health disparity when it comes to T2D. The blue shaded part of the infographic above shows the relationship between race and diabetes cases and education and diabetes cases in the US between 2017 and 2018.
American Indians have the highest prevalence for T2D in the US followed by Black and Hispanic population groups. White Americans are the least affected.
Looking at education Americans who did not complete highschool have almost double the prevalence (all cases diagnosed) than Americans with at least some college.
T2D Is Increasingly Seen in Younger Populations
T2D was always a disease of middle and old age and T2D diagnosis in teens was almost unheard of. Lately this perception needs to be revised. Today, T2D diagnosis is spreading to younger age groups as well.
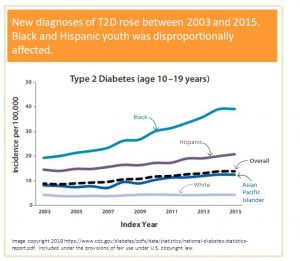
When you look at the graph on the left you see a doted line. This line depicts the number of total new T2D cases diagnosed each year between 2003 and 2015. You see a slow continuous rise in new diagnoses over those years. When you look at the blue top line depicting new diagnoses in Black teens the increase is staggering.
In 2015 38 Black teens out of 100 000 were diagnosed with T2D and that seems at first look relatively low. The scary part is the upward trend and the clear health disparity. While white American youth is mostly unaffected, T2D in Black Youth increases rapidly. A more modest rise is also seen in Hispanic and Asian teens.
The data tells us that there is a trend that needs to be reversed before the situation gets further out of control.
T2D Develops As a Consequence of Obesity, Lifestyle and Genetic Predisposition
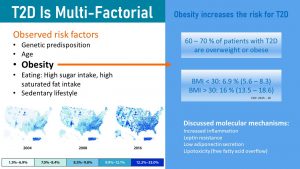
The main factor determining the risk for T2D is obesity. As you learned in chapter 1 obesity, especially the visceral type, increases chronic, systemic inflammation. Circulating cytokines act on all cells in the body. When it comes to the glucose metabolism cytokines disrupt insulin signaling and impair insulin production in the beta-cells of the pancreas. In addition, low adiponectin and high leptin secretion by the excess adipose tissue also change the regulation of the glucose metabolism.
Scientifically we understand many factors that aggravate the metabolic effects of obesity and will ultimately lead to type 2 diabetes. Modifiable risk factors increasing the risk for T2D are a sedentary lifestyle, a high intake of sugars and saturated fat, the modern processed food diet. But, not everybody with an unhealthy lifestyle will develop T2D.
Genetic predisposition and age are the two non-modifiable risk factors that determine if an unhealthy lifestyle will result in T2D or not. Vice versa a healthy lifestyle can prevent the development of T2D even if the genetic risk is high. For example, if all risk factors come together a teen might already develop T2D. If a person has a genetic predisposition but lives a healthy, active lifestyle T2D might not develop at all or only in high age. Some people develop pre-diabetes but never progress to a T2D diagnosis. Others develop T2D but can reverse it.
When you study the development of T2D keep in mind that for every diagnosed person the course of this chronic disease varies widely.
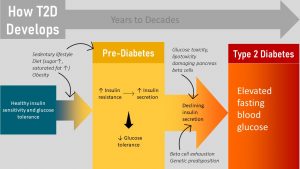
Type 2 Diabetes Develops Over Years to Decades
T2D Development Is Complex and Highly Individual
T2D is often portrait as insulin resistance that can be reversed with lifestyle interventions. In reality T2D is a much more complicated disease. Per definition T2D is the inability of the pancreas beta cells to increase insulin production sufficiently to compensate for progressive insulin resistance. Let this sentence sink in.
There are two metabolic changes going on:
- Insulin resistance: T2D starts out when insulin sensitive cells (muscle, liver, adipose tissue) become less sensitive to the hormone insulin. Insulin becomes progressively inefficient to unlock those insulin sensitive cells for glucose uptake.
- Inadequate insulin production by the pancreas beta cells: While the development of T2D starts with insulin resistance over time the beta cells of the pancreas are first forced to produce increasing amounts of insulin which might be followed by a decline of insulin production.
How fast T2D develops and how severe it will become is highly individual and depends on the genetic predisposition and lifestyle. Most genetic variants increasing the risk for T2D affect beta-cell function. For example when insulin resistance due to systemic inflammation it is compounded by an impaired beta-cell function due to a genetic predisposition. While studying the next section, keep in mind that disease progression will vary from person to person. Some individuals will go through the stages within years while others will stop progression at one point and not develop more severe T2D.
Let’s have a look at the stages of T2D development:
1. Healthy Blood Glucose Regulation
When healthy people eat carbohydrates the synthesis of insulin in the beta-cells of the pancreas is triggered. Glucose is detected in the stomach and small intestine during digestion by receptors and hormones called incretins are secreted into the blood circulation. These hormones announce that glucose will soon be absorbed. The beta cells of the pancreas react to this hormonal message by producing and secreting a first wave of insulin to get cells ready for glucose metabolism.
Once the absorption of glucose starts and glucose enters the bloodstream the second wave of insulin secretion is triggered. Glucose molecules dock at receptors in the pancreas and increase the insulin secretion depending on the blood glucose concentrations. This is a finely tuned mechanism.
In individuals with a healthy insulin response and glucose metabolism postprandial blood glucose is usually removed within 60 minutes to 2 hours depending on the meal.
Carbohydrates from a meal high in fiber and fat with a low glycemic index will stream slower into the blood circulation. The blood glucose concentration is lower but longer elevated. On the opposite end drinking sugary soda will cause a steep spike in blood glucose that is faster removed. Once the blood glucose is back to normal circulating insulin is deactivated and the beta cells reduce the production and secretion of insulin.
2. Development of Insulin Resistance, Hyperinsulinemia, Impaired Glucose Tolerance (IGT)
How does T2D develop? Nobody wakes up one morning and all of a sudden has T2D. Instead T2D is a chronic disease that develops over years to decades. The following graph focuses on pre-diabetes and depicts the interaction between metabolic activity (insulin resistance, insulin secretion, beta cell function) and the corresponding glucose regulation (fasting glucose and postprandial glucose).
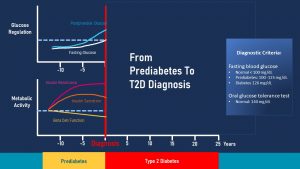
The start of T2D is insulin resistance depicted by the magenta line in the metabolic activity portion.
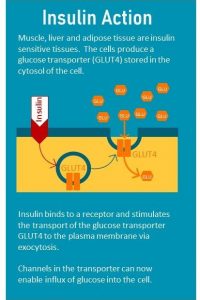 When the cell is insulin sensitive, insulin docks to the receptor located in the cell membrane which starts a cascade of signals that ultimately lead to the the translocation of the glucose transporter from the cytosol to the cell surface.
When the cell is insulin sensitive, insulin docks to the receptor located in the cell membrane which starts a cascade of signals that ultimately lead to the the translocation of the glucose transporter from the cytosol to the cell surface.
When people develop insulin resistance this signaling cascade is disrupted and insulin becomes less efficient to translocate the glucose transporter to the cell membrane. The glucose transporter stays in the cytosol and insulin loses it’s ability to unlock the glucose influx into the cell.
The pancreas reads the inefficient removal of glucose after a meal as a cue that not enough insulin was secreted and tries to correct the situation by secreting even more insulin. If enough insulin is secreted to compensate for the insulin resistance glucose is slowly removed from the blood stream.
Insulin blood levels are now elevated which is called compensatory hyperinsulinemia (orange line in the graph above).
If blood glucose concentrations are tested at this early point, fasting and post-prandial blood glucose concentrations are within normal ranges (white and light blue lines in the glucose regulation portion of the graph.)
As insulin resistance increases, insulin production keeps increasing until the point is reached when the beta cells of the pancreas cannot increase production any further. As a consequence, it will take longer and longer to remove glucose from the blood circulation after a meal. This is called impaired glucose tolerance (IGT).
At this point an oral glucose tolerance test testing the postprandial glucose response will come back elevated (blue line). A fasting glucose test will still be within normal range or might be slightly elevated above 99 mg/dl but still under 125 mg/dl. This is often the point when pre-diabetes is diagnosed during a check-up.
Are you at risk for pre-diabetes? Take the CDC/ADA test: https://www.cdc.gov/diabetes/risktest/inttps://www.cdc.gov/diabetes/risktest/index.html
3. The Road to the T2D Diagnosis
In summary, a person with pre-diabetes has an increased insulin resistance, hyperinsulinemia, elevated postprandial blood glucose and a fasting blood glucose slightly higher than normal. Many people remain in this stage or reverse the metabolic changes after the pre-diabetes is detected.
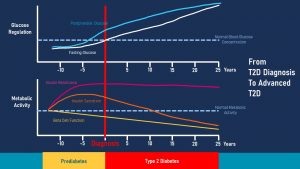
At this point the beta cells of the pancreas have to work overtime after each meal. Imagine pancreas beta cells as a motor. If the motor runs constantly on high gear it will exhaust faster. When the function of the beta cells declines due to overworking it is called beta cell exhaustion (yellow line in the metabolic activity section.)
In addition, the prolonged high concentrations of glucose after a meal are toxic to cells and will damage the beta cells of the pancreas aggravating the progressing beta cell exhaustion.
Over time a combination of genetic pre-disposition, glucotoxicity and beta cell exhaustion will cause more and more individual beta-cells to stop functioning and undergo cell death. Since these dying beta-cells are not replaced beta cell mass in the pancreas keeps declining and insulin production starts to decline as well.
The insulin secretion is not able to compensate for the insulin resistance any longer and blood glucose levels will remain elevated at all times. This is the point when a yearly check-up will find elevated fasting blood glucose levels over 125 mg/dl. This is the diagnostic criterium for T2D.
Think back to the review at beginning of the chapter (or scroll up and have a quick look). Another function of insulin is to suppress lipolysis, the release of fat from the adipose tissue. Low insulin blood levels will remove the break on lipolysis and result in elevated free fatty acids in the blood stream. High free fatty acids and high glucose concentrations are both toxic to cells. This toxicity will speed up beta cell apoptosis and with that insulin production will further decline and T2D will progress.
At one point the beta cell mass is so much reduced that the person with T2D will become irreversibly insulin dependent for life.
Here is where age comes in. Most people lose beta cells physiologically as they age, even with a healthy lifestyle. In addition systemic inflammation increases slowly with old age. This is the reason why age is a non-modifiable risk factor for T2D.
Chronic Systemic Inflammation Disrupts Insulin Signaling and Damages Beta-Cells
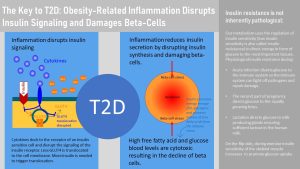
How is chronic systemic inflammation connected to insulin resistance and T2D? As the adipose tissue, especially visceral adipose tissue, enlarges and becomes inflamed, macrophages secrete cytokines into the blood circulation. When cytokines dock at receptors of insulin sensitive cells, such as muscle, liver and adipose tissue cells, they disrupt the insulin signaling cascade. As a result more insulin is needed to compensate for the decreased ability of insulin to trigger glucose uptake into the cell (compensatory hyperinsulinemia.) This disruption reduces sensitivity to insulin also called increased insulin resistance.
The cytokines also trigger inflammation in other tissues (systemic inflammation means in all tissues.) This includes the pancreas. As a result of pancreas inflammation beta-cells undergo stress or even die. Insulin production declines. The combination of increasing insulin resistance and declining insulin production in the beta-cells causes a decrease glucose tolerance—glucose blood levels remain elevated longer after a meal—and over time T2D, elevated glucose at all times.
The question is now why insulin resistance progresses to reduced glucose tolerance and why glucose intolerance progresses to T2D. Recall that insulin has other metabolic functions than unlocking cells for glucose influx. Insulin promotes energy storage after a meal and suppresses the release of fatty acids from the adipose tissue. Once the adipose tissue becomes insulin resistant the break is removed from lipolysis and the adipose tissue releases large amounts of free fatty acids into the circulation. If liver cells become insulin resistant they oxidize less fat and synthesize more fatty acids, releasing also more free fatty acids.
As a consequence blood levels of free fatty acids increase. The combination of beta-cell stress from inflammation and glucolipotoxicity fuels the progression of T2D. Reversal of insulin resistance, glucose intolerance or T2D will require the reduction of systemic inflamation. This can be achieved with lifestyle changes including weight loss but few people with prediabetes or T2D are successful changing their lifestyle. The goal of oral medications is to prevent complications but studies show that not all complications can be prevented unless inflammation is reduced. Research today is focusing on medications that can reduce inflammation substantially.
Intervention Often Starts When Damage Is Already Done
In the past it was thought that catching pre-diabetes and early T2D is a good point to start interventions. It has become clear though that even the metabolic changes during often undiagnosed insulin resistance will damage blood vessels and contribute to CVD. This means that lifestyle interventions should take place as early as possible.
T2D Complications Are Dreaded Consequences Of Glucose And Free Fatty Acid Toxicity
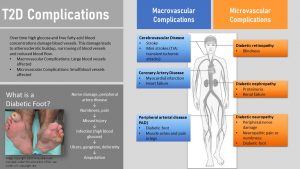
Glucolipotoxic damage starts already during insulin resistance and pre-diabetes. Both, elevated blood concentrations of glucose and free fatty acids are cell toxic and affect every tissue they come into contact with.
You already learned about the damage to beta cells. The glucolipotoxicity also damages the lining of large blood vessels and capillaries spreading into organs and neurons.
Initially, there will be mostly damage to major arteries resulting in macrovascular complications. Macrovascular complications start with damage to the epithelial lining of major blood vessels. This speeds up atherosclerosis resulting in an increased risk for heart attack and stroke in patients with T2D.
Over time smaller blood vessels are increasingly damaged leading to microvascular complications.
Blood vessels carry nutrients to neurons and over time glucolipotoxicity will damage the nerve endings of those neurons. Diabetic neuropathy develops, a loss of feeling leading to numbness in extremities.
Legs and feet are often affected. A pebble in the shoe or a shoe causing a blister will not be noticed in time. Due to ample availability of glucose in the injured tissue bacteria can grow rapidly and infect the foot. Reduced blood circulation due to damage of blood vessels in the extremities slows down and prevents healing. This small injury can lead to diabetic foot, gangrene and ulcers in the foot, and ultimately if not treated successfully to amputation.
Damage to neurons can also lead to problems with the digestive system, urinary tract, blood vessels and heart.
Damage to the eyes can lead to blindness (diabetic retinopathy) while damage to small blood vessels in the kidney can ultimately lead to high blood pressure, kidney damage and failure.
In addition to the well-researched T2D complications latest research also suggests that T2D contributes to the progression of Alzheimer’s disease, non-alcoholic fatty liver disease, gout and rheumatoid arthritis.
Early Metabolic Changes Cannot Be Detected Efficiently
Understanding the development of T2D and the development of T2D complications, it is clear that interventions need to start as early as possible: Ideally at the beginning of insulin resistance, but at the minimum before beta cells exhaustion takes place or beta-cell mass is irreversibly reduced.
Unfortunately pre-diabetes and early T2D are silent diseases and do not cause any symptoms.
Only after T2D has progressed for years and insulin production is severely limited symptoms will appear.The symptoms of advanced T2D include profound exhaustion, hunger, increased urination and thirst, slow healing of wounds, tingling and numbness in extremities. It will take years to get to this point and macro- and microvascular damage are already well underway.
The second problem causing a later diagnosis than we would like is that we do not have easy lab tests to detect insulin resistance and early IGT at a physician’s office or at public health screening events. If people have health insurance and attend regular check-ups problems are most likely detected if a fasting blood glucose test comes back elevated. This is not ideal.
Low-income populations might have no or only insufficient health insurance or they might not have the time and money to attend regular check-ups. These groups are more likely diagosed after severe symptoms prevent daily functioning. This explains in part the health disparities explained at the beginning of the chapter. The opportunity to catch pre-diabetes to start interventions is missed and full T2D develops.
To understand this lets look at the metabolic parameters that can be measured:
- Insulin blood levels: Not a routine test; only used to monitor diabetes and insulin resistance in a clinical, scientific setting.
- Post-prandial blood glucose: An oral glucose tolerance test measures blood glucose concentration 60 minutes or 2 hours after drinking a glucose solution; this is not a routine test and is more likely ordered if the physician suspects the development of T2D.
- Finger prick test: Measures blood glucose concentration quickly and is often completed at the beginning of a checkup. Might be able to indicate a potential problem after IGT has developed.
- Fasting blood glucose: Blood glucose measured after an overnight fast of 8 hours; this test is part of screening tests at check-ups starting in middle age.
- A1C test : Measures the glycosylation of erythrocytes and can monitor glucose control in diabetes; not precise enough to detect insulin resistance early but can detect it once insulin resistance progresses; not a routine check-up test.
While we would like to detect issues with glucose regulation as early as possible to prevent macrovascular damage, routine tests are not as efficient as we would like. Even more importantly, the tests we have are too often not accessible to low-income populations and people who do not have regular check-ups. Early lifestyle interventions could prevent progression of pre-diabetes into T2D and the progression of early, reversible T2D into irreversible, insulin dependent T2D.
Lifestyle Interventions For Pre-Diabetes And T2D Include a Plant-Heavy Eating Pattern, an Active Lifestyle And an Exercise Program
Understanding the development of T2D and the consequences make it clear that changes in glucose metabolism need to be detected as soon as possible so intervention can start. Only if beta-cell function is adequate the patient will be able to fully reverse the disease.
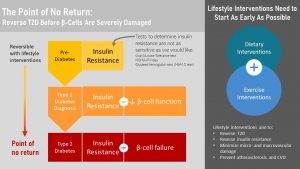
Keep in mind that not every person diagnosed with pre-diabetes will progress into full T2D. About 10 – 20 % of people diagnosed with pre-diabetes will progress to T2D. But, even during pre-diabetes macrovascular damage will take place and the risk for heart attacks and stroke will increase.
If pre-diabetes is diagnosed lifestyle interventions will aim for weight loss by implementing lifestyle changes. Even a small weight loss of 5 – 10 % will improve metabolic health. Larger weight loss and an active healthy lifestyle can reverse insulin resistance completely.
If people have trouble losing weight and metabolic health cannot be fully restored the goal is to reduce insulin resistance as much as possible to prevent progression into full T2D and to limit macrovascular damage.
Once T2D is diagnosed the treatment has three goals:
- Weight loss to improve insulin resistance and prevent further decline in beta cell function and beta cell failure
- Management of blood glucose to prevent glucotoxicity (macro- and microvascular damage)
- Prevention of CVD (heart attack, stroke)
The point of no return is reached when beta-cell failure sets in. At this point beta-cell mass is so much reduced that T2D cannot be fully reversed. Lifelong insulin injections will become necessary. Lifestyle interventions are still essential for optimal blood glucose management which will prevent the dreaded micro- and macrovascular damage and diabetic complications. Improved insulin resistance due to weight loss will keep the injected insulin dose low. This is important becaise hyperinsulinemia regulates other tissue function. Normal insulin lblood levels are necessary for example for healthy brain function.
The Pillars of T2D Dietary Intervention Are a Nutrient-Dense Plant Heavy Eating Pattern Limiting Added Sugar and Saturated Fat Intake
Insulin resistance is improved or reversed by losing fat mass and increasing muscles mass. While muscle mass is increased by exercise, especially strength training, weight loss will require dietary intervention. The weight loss should be slow and gradual. The recommended approach is a plant-heavy diet while energy intake should be reduced moderately by 300 to 500 kcal.

When you read online articles or even discussions between physicians, an array of recommended dietary patterns reaching from keto diet, low-carb diet, Paleo diet, vegetarian and vegan diets are passionately discussed. These discussions often are only backed by personal experience and less by sufficient scientific evidence.
Based on the existing scientific evidence the diet should include lots of fruits and vegetables, whole grains, legumes, dairy, lean meat and fish. While very similar to the general recommendations for a healthy diet the T2D dietary pattern tends to be lower in carbohydrates and is definitively low in added sugar.
Dietary fat composition needs to be shifted from saturated fats to monounsaturated and polyunsaturated fats. This has two goals. Polyunsaturated fats have been shown to increase insulin sensitivity. Secondly, this healthy dietary fat profile is a prevention measure for CVD since people with T2D have an increased risk.
Some physicians will recommend a low-carb diet once T2D is diagnosed. The idea behind this is that low carbohydrate intake reduces the need for insulin during and after a meal. This is controversial because other studies show that the intake of soluble fiber from fruits, whole grains and legumes slows down the glucose uptake during absorption and is beneficial for the management of blood glucose levels. Fiber from whole grains and legumes have a beneficial effect on insulin sensitivity.
New studies suggest that for some patients with low insulin production the Keto-diet might be beneficial. The evidence is limited and the extreme Keto diet is hard to adhere to long-term. This type of diet should only be reserved as a last resort if no other interventions work and must be conducted under the supervision of a physician to ensure good blood glucose management and avoid hypoglycemia.
Overall, the scientific evidence does not support a specific diet for T2D. Some studies see improved insulin resistance and better blood glucose management using a high carbohydrate/low-fat diet. Other studies find similar results for a low-carbohydrate diets or a high protein diet. The DASH and Mediterranean diets are also discussed as beneficial.
Intervention studies for keto, low-carb, Mediterranean, DASH and vegetarian diets show benefits. The main issue is that these intervention studies are often short-term. A dietary pattern needs to work long-term though which means good adherence needs to be achieved.
Therefore a variation of the plant-heavy diet with a healthy fatty acid composition should be chosen based on what eating pattern an individual with T2D will enjoy and therefore tolerate long-term.
Keep in mind that these recommendations do not apply to advanced T2D requiring insulin. Here medical nutrition professionals need to work with the patient on a personalized approach.
Many questions regarding dietary intervention are still open and will need much more research. Some of those questions are:
- Would a carbohydrate intake under 45 % energy (low carb diet) aid weight loss and prevent T2D? How low should the carbohydrate intake be? At what point is the carbohydrate too low for good patient adherence?
- Does ketogenesis generated by extreme carbohydrate restriction (keto diet) have unique metabolic advantages in patients with T2D? Can patients adhere to the keto-diet? Would the keto diet be advisable long-term?
- Do fructose sweeteners (HFCS) cause metabolic benefits or harm? Recent research suggests that high fructose intake might damage the gut barrier, contributing to inflammation.
- Would replacing whole grains with fiber-rich fruits and vegetables have additional benefits?
Physical Activity Intervention Should Go Hand In Hand With Dietary Intervention

Dietary intervention should go hand and hand with a physical activity program. The health benefits for individuals with pre-diabetes or T2D at any stage are three-fold:
- Improved insulin resistance
- Better blood glucose management
- CVD prevention
Increased muscle mass is associated with reduced insulin resistance. Therefore individuals with impaired glucose tolerance and T2D should start a strength training program.
All physical activity and regular exercise will help manage blood glucose concentrations. While the muscle needs insulin to take up glucose efficiently in a sedentary state, during exercise glucose is taken up readily by the muscle cell using an insulin independent pathway. It is thought that the increased need for glucose by the muscle cell triggers molecular pathways that bypass the insulin resistance and increase the number of glucose transporters in the cell membrane. As a consequence the glucose uptake in the muscle is at normal or near normal levels during exercise.
As an additional benefit, insulin sensitivity increases after exercising allowing for more glucose uptake. These two mechanism allow for better blood glucose management.
Regular, moderate aerobic exercise, as you will learn in the CVD chapter in more detail, is one of the best CVD prevention tools. Exercise reduces blood pressure, blood lipids are shifted into a more beneficial pattern, chronic inflammation is reduced, and blood vessel endothelium is healthier. Since individuals with T2D are at a higher risk for CVD due to macrovascular complications regular aerobic exercise is essential.
How much exercise is necessary? Do all people with a T2D diagnosis need to start running marathons like Chris Peck? Keep in mind that many people with pre-diabetes or T2D tended to lead a sedentary life and are often obese. Going from sedentary to an athletic lifestyle will usually not happen. Luckily studies show that this is not necessary.
The recommendation is a combination of moderate aerobic exercise and strength training on most days of the the week for 30 to 60 minutes. A brisk walk every day for 30 minutes along with strength training will improve metabolic health. In addition patients should become aware of their level of general physical activity and aim to include an active lifestyle as much as possible.
Is Bariatric Surgery The Solution?
Reading through the section, prevention and interventions for T2D seem very reasonable and doable. On paper. The interventions seem to be all common sense and a weight loss of 5 to 10% does not sound huge.
From scientific studies and practical experience we know that it is not that easy. There are many challenges to treating T2D in obese people. Interventions tend to stimulate weight loss initially but the key problem is maintaining this weight loss long-term.
People with T2D are monitored using the A1C test at regular check-ups. The A1C reflects how well the blood glucose was managed over the last 2 to 3 months. Most people treated for T2D don’t meet the goal for glycemic control, an A1C under 7%.
Once it becomes clear that T2D cannot be controlled by lifestyle interventions alone people start taking antidiabetic drugs mediating insulin resistance or insulin secretion. These drugs bring a risk of hypoglycemia if the patient does not eat enough orb is exercising vigorously.
This is the point when patient and physician start discussing bariatric surgery.
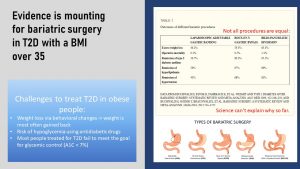
Bariatric surgery was developed for rapid weight loss in severely obese patients. The idea was to reduce the stomach volume so the person feels full fast during a meal. Smaller meals, the scientists hypothesized, will translate into less energy intake leading to rapid weight loss.
The hope was that, once the weight is lost, the patient would be metabolically healthier and conditions such as T2D would go into remission. To the surprise of physicians and scientists the improvement of insulin resistance and T2D was immediate after the surgery before weight was lost. Over time many patients saw complete remission.
It is clear that in addition to weight loss something else is changing after surgery. The leading hypothesis right now centers around the group of incretin hormones. Incretins are secreted into the blood stream after carbohydrate in food enters the stomach. When incretins reach the pancreas they prepare the metabolism for the incoming glucose by triggering the release of insulin. This mechanism helps regulate blood glucose after a meal more efficiently.
Incretin secretion from the stomach is lower in obese individuals leading to greater blood glucose spikes after a meal. One type of gastric bypass surgery leads the majority of food directly into the small intestine. The small intestine also has the ability to secrete incretins. Leading the food more directly into the small intestine triggers a good incretin response independent of the weight status. As a consequence post-prandial blood glucose is better regulated after a meal. This is early research though and other factors might play a role as well.
Coming back to Chris Peck, the marathon runner. What looks so easy in the article is very difficult for many people struggling with obesity and T2D. Reversing pre-diabetes or T2D will require weight loss triggered by a healthy lifestyle that includes a plant-heavy eating pattern, moderation of processed food consumption rich in added sugar and saturated fat, combined with an active lifestyle and an exercise program.
This sounds easy, but is for most people struggling with obesity this will be a complete reversal of their lifestyle and hard to maintain. As you will learn throughout this OER reversal of chronic diseases is extremly hard and chronic diseases are best prevented starting as early as possible in life.
Interested In More Information?
- Bell KJ, Colagiuri S, Brand-Miller J. Diabetes and insulin resistances. In: Marriott BP, Birt DF, Stallings VA, Yates AA eds. Present Knowledge in Nutrition: Volume 2: Clinical And Applied Topics in Nutrition. London, United Kingdom: Elsevier; 2020:361-377.
- National Diabetes Statistics Report 2020 (CDC): https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- Diabetes In Other Countries (WHO): https://www.who.int/diabetes/country-profiles/en/
- If you are interested in the connection between obesity, inflammation, T2D and other chronic diseases, here is a good, recent review: Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55(1):31-55. doi:10.1016/j.immuni.2021.12.013
Editor: Sydney Christensen
NUTR251 Contributors:
- Spring 2020: Audrey Freyhof, Rawan AL Jabri, Maddison Korthas, Kenyon Gaar, Terrell Horton, Eli Havekost, Jenna Junker, Dario Henry, Brett Freitag, Danae McKenzie
Incretins are metabolic hormones that are secreted from gut cells into the blood within minutes after eating. One of those incretins is GLP-1. GLP-1 increases the amount of insulin that is secreted after eating even before glucose is absorbed. Other functions of GLP-1 are reducing glucagon secretion, delaying gastric emptying and suppressing appetite.
After a meal
transport of the transporter from the cytosol where it is produced and stored to the cell membrane
Measures the metabolic reaction to glucose. It is used if IGT is suspected. Blood is drawn to determine to glucose concentration before the test. Then the patient drinks a glucose solution. If the blood glucose is still elevated after 2 hours IGT or pre-diabetes is diagnosed.
cell death
Impaire glucose tolerance
In nondiabetic individuals, plasma glucose concentrations start to rise 10 minutes after the beginning of the meal and peak around 60 min. Blood glucose concentrations rarely exceed 140 mg/dl, and return to preprandial levels within 2–3 h. Even though glucose concentrations have returned to preprandial levels by 3 h, absorption of the ingested carbohydrate continues for at least 5–6 h after a meal.
Hemoglobin A1C test (A1C test for short) reflects the glucose blood levels over the last 2 to 3 months. Some of the glucose circulating in the blood binds to hemoglobin. If blood glucose levels are high—in advanced pre-diabetes they would be longer elevated after a meal—more glucose binds to hemoglobin. The percentage of glycated hemoglobin in the blood will be higher.
Bond forming between a glucose molecule and a protein for example
low blood glucose concentrations


Feedback/Errata